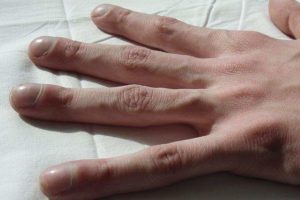
EMA grants orphan designation to selumetinib to treat neurofibromatosis type 1
AstraZeneca licensed selumetinib from Array BioPharma in 2003. AstraZeneca and Merck have entered a co-development and co-commercialisation agreement for selumetinib in 2017. NF1 is an incurable genetic condition,
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.