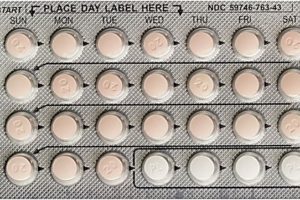
Janssen Pharmaceuticals is voluntary recalling one lot of Ortho-Novum 1/35 (norethindrone/ethinyl estradiol) tablets and two lots of Ortho-Novum 7/7/7 (norethindrone/ethinyl estradiol) tablets to the pharmacy level.
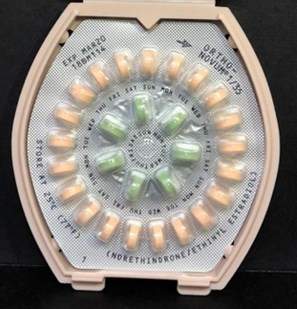
Image: Ortho-Novum 1/35 (norethindrone/ethinyl estradiol) tablets. Photo: courtesy of PRNewswire / Janssen Pharmaceuticals, Inc.
Subscribe to our email newsletter
The ORTHO-NOVUM product itself remains safe and effective for use with the appropriate dispenser instructions. Women should continue to take the 21 “active” pills (with hormones) (peach for ORTHO-NOVUM 1/35; white, light-peach and peach for ORTHO-NOVUM 7/7/7) for three weeks, followed by the one week of green “reminder” pills (without hormones).
The three lots affected by this recall are:
|
Product Description |
NDC Number (carton) |
NDC Number (pouch) |
Lot No. |
Expiration Date |
|
ORTHO-NOVUM 1/35 |
50458-176-06 |
50458-176-28 |
18BM114 |
03/2020 |
|
ORTHO-NOVUM 7/7/7 |
50458-178-06 |
50458-178-28 |
18CM120 |
03/2020 |
|
ORTHO-NOVUM 7/7/7 |
50458-178-12 |
50458-178-12 |
18BM110 |
03/2020 |
This recall only affects the U.S., and no other ORTHO contraceptive products beyond the three lots listed above are impacted by this recall action. ORTHO TRI-CYCLEN LO, ORTHO TRI-CYCLEN, ORTHO MICRONOR and ORTHO CYCLEN are not affected.
Products were distributed in the U.S. to wholesalers, distributors and pharmacies, and they have been notified by recall letter to return affected products.
Consumers with ORTHO-NOVUM product from the affected lots can access the correct instructions for the Veridate dispenser pack at https://www.janssen.com/us/our-products and talk to their prescribing medical professional if they have any concerns. Consumers should not stop taking the product and if they do miss a dose, they should follow the instructions included in the packet.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Source: Company Press Release.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.