Celltrion Healthcare has secured approval from the European Commission (EC) for its Remsima SC (CT-P13 SC), a subcutaneous formulation of biosimilar infliximab, Remsima, to treat patients with rheumatoid arthritis (RA).
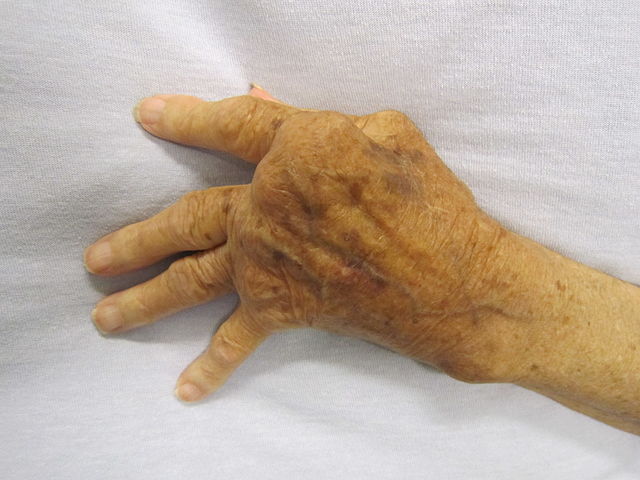
Image: A hand severely affected by rheumatoid arthritis. Photo: courtesy of James Heilman, MD.
Subscribe to our email newsletter
Remsima SC is claimed to be the world’s first subcutaneous formulation of infliximab.
The EC approval was based on clinical evidence, including results from a study that demonstrated switching people with RA from the intravenous (IV) formulation to the subcutaneous (SC) formulation of CT-P13 treatment at week 30 was comparable to maintaining CT-P13 SC up to week 54.
The patients themselves can inject the Remsima SC, helping to reduce hospital visits and save time normally need for hospital-administered IV treatment.
In the EU, the Remsima SC, in combination with methotrexate (MTX), was approved to treat adult patients with active RA when the response to disease-modifying anti-rheumatic drugs (DMARDs), including MTX, has been inadequate.
Remsima SC was also approved to treat adult patients with a severe, active and progressive disease not previously treated with MTX or other DMARDs.
The company has also submitted a further variation to the marketing authorisation of Remsima SC to expand the indication of inflammatory bowel disease. Celltrion is expecting the approval decision in mid-2020.
The company is planning to introduce Remsima SC across Europe in the first quarter of 2020. In addition, the firm applied for patent protection for Remsima SC until 2038 in around 100 countries across the US, Europe and Asia.
The CT-P13, which was developed and produced by Celltrion, is said to be the world’s first monoclonal antibody biosimilar approved by the EC. It was approved to treat eight autoimmune diseases, including RA and inflammatory bowel disease.
Celltrion Healthcare vice-chairman Hyoung-Ki Kim said: “The development of Remsima SC™ demonstrates that Celltrion Healthcare is not just a biosimilar company, it is also an innovative company that strives for novel solutions such as the SC formulation of biosimilar infliximab.
“We develop cost-effective and patient-centered treatments to enable more patients to gain access to biologics that have proven efficacy and safety profiles.”
In December 2018, Teva Pharmaceutical Industries, along with Celltrion, secured approval from the US Food and Drug Administration (FDA) for breast cancer drug Herzuma (trastuzumab-pkrb), a biosimilar to Roche’s Herceptin.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.

