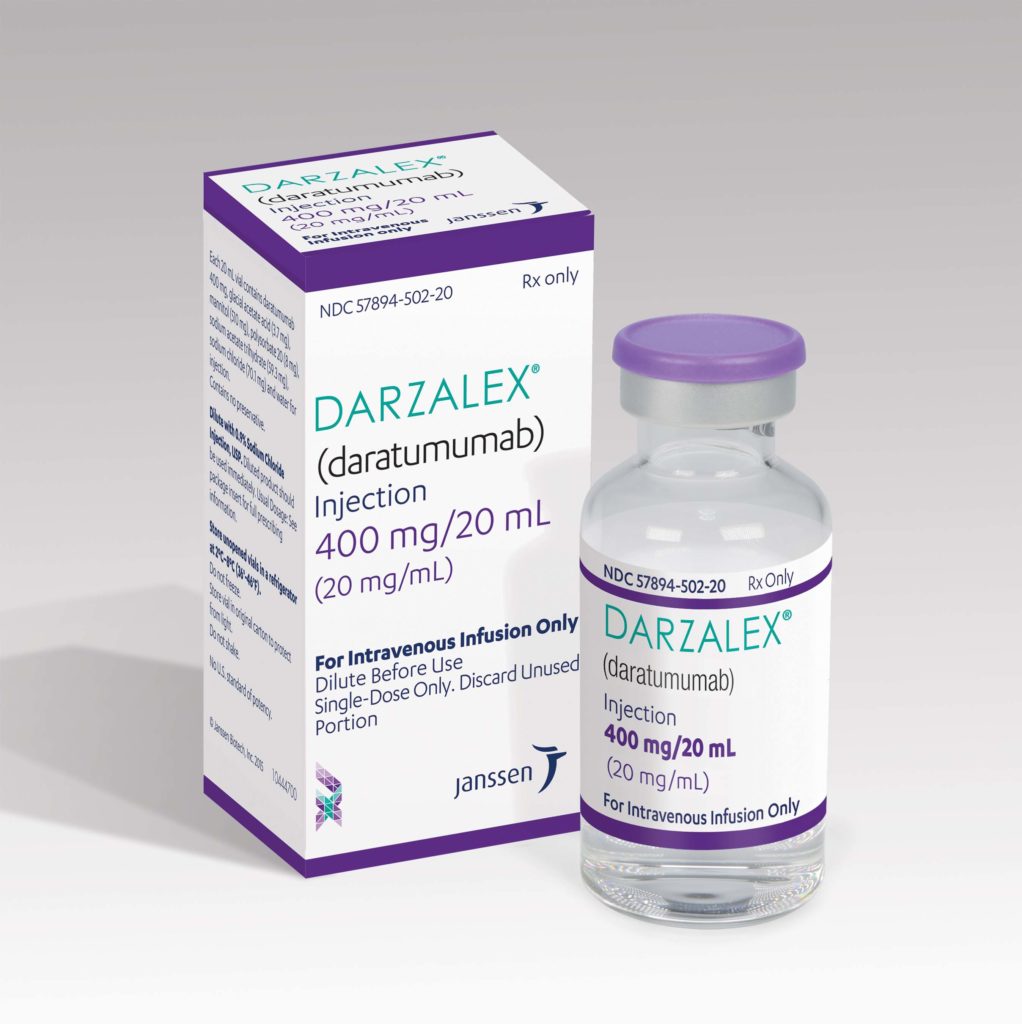
SC formulation of DARZALEX combination delivers deep and rapid haematologic responses and improved clinical outcomes in newly diagnosed light chain amyloidosis
The Janssen Pharmaceutical Companies of Johnson & Johnson announced today results from the first randomised Phase 3 study investigating the subcutaneous (SC) formulation of DARZALEX (daratumumab) in the treatment of patients with newly diagnosed light chain (AL) amyloidosis, a rare and potentially fatal disease.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.