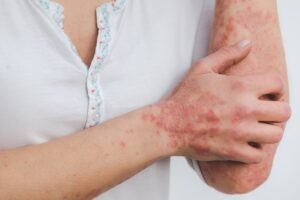
Azitra’s ATR-04 receives FDA fast track status for treating skin rash
This topical therapy is aimed at treating moderate to severe skin rash. Azitra CEO Francisco Salva said: “Many cancer patients receive EGFR inhibitors, which often have significant side
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.