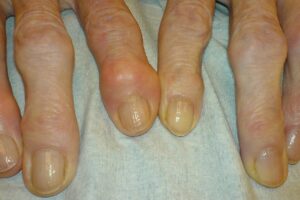
4P-Pharma spin-off 4Moving Biotech (4MB) has received €7.6m ($8.3m) in funding from the France 2030 plan to expedite the clinical development of 4P004 for knee osteoarthritis, currently in a Phase IIa clinical trial.
The trial's main goal is to assess a single intra-articular injection of 4P004 in reducing knee pain. Credit: Markus Spiske by Unsplash.
Subscribe to our email newsletter
France 2030 is a selective French government initiative to fast-track “breakthrough innovations”.
The funding is an endorsement of 4MB, achieved through a multi-stage assessment by experts in science, clinical and technology fields.
It is stated to hasten the INFLAM MOTION Phase IIa clinical study of 4P004, a GLP-1 analog that aims to provide anti-inflammatory, analgesic, anti-catabolic, and anabolic effects for joint tissues.
The trial’s main goal is to assess the efficacy of a single intra-articular injection of 4P004 in minimising knee pain.
It will also evaluate structural enhancements using contrast-enhanced MRI and investigate new biomarkers that indicate disease progression and the potential to postpone total knee replacement.
4MB CEO Luc Boblet said: “4MB gains extended momentum through The France 2030 grant marks an important milestone in the 4MB roadmap.
“With our phase IIa already initiated, this funding strengthens the value of our work, from early-stage discovery to clinical stage, and highlights the need to bring transformative therapies for patients suffering from OA”.
With France 2030’s backing, 4MB is stated to be in a position to deliver clinical proof-of-concept in a timely manner.
This milestone is crucial for advancing to the next development phase and demonstrating 4P004’s potential as a disease-modifying drug (DMOAD), which may lead to future partnerships.
The France 2030 initiative is being implemented by the French Agency for Ecological Transition (ADEME), the French National Research Agency (ANR), Bpifrance, and the Caisse des Dépôts et Consignation (CDC).
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.