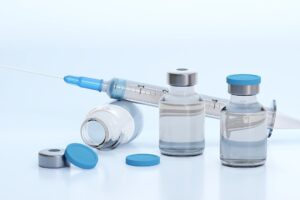
Amneal Pharmaceuticals has initiated the distribution of its over-the-counter (OTC) Naloxone Hydrochloride (Naloxone HCI) Nasal Spray, USP, 4mg, to retail pharmacies across the US, including the State of California.

Amneal Pharmaceuticals develops, manufactures and distributes over 280 pharmaceutical products. Credit: Ri Butov from Pixabay.
Subscribe to our email newsletter
The product, which received approval from the US Food and Drug Administration in April 2024, is now available for direct distribution to states and municipalities nationwide.
Naloxone HCI Nasal Spray rapidly reverses the effects of life-threatening opioid emergencies. By binding to opioid receptors in the brain, it blocks the effects of opioids, potentially restoring normal breathing within two to three minutes in individuals experiencing an overdose from opioids such as heroin, fentanyl, and prescription medications.
Amneal’s Naloxone HCI Nasal Spray is the generic equivalent to OTC NARCAN HCI Nasal Spray, a medication for the treatment of opioid drug overdoses. NARCAN is a registered trademark of Emergent Operations Ireland Limited.
Amneal has also entered into a distribution agreement of Naloxone HCI Nasal Spray with California through the CalRx Naloxone Access Initiative.
Looking ahead, Amneal anticipates a significant increase in production capacity. The company expects to produce approximately ten million two-packs annually at its New Jersey manufacturing facility starting in 2025, ensuring a steady supply to meet the critical demand for this emergency treatment.
Amneal Generics chief commercial officer and executive vice president Andy Boyer said: “Amneal is an industry leader in complex generics with the ability to develop, manufacture and commercialise high-quality medicines that are affordable and accessible. The widespread availability of OTC naloxone will be a critical tool in the fight against the ongoing opioid epidemic. Amneal is proud to be part of the solution.”
Amneal SVP for Regulatory Strategy and Government Affairs Maryll Toufanian said: “We are pleased to partner with the State of California on a long-term distribution agreement to make OTC naloxone available to thousands of communities and millions of people. We are actively engaging with other states and municipalities to expand access to this important life-saving product, which is proudly made in America in one of our New Jersey facilities.”
Headquartered in Bridgewater, New Jersey, Amneal Pharmaceuticals is into the development, manufacturing, and distribution of a varied portfolio of over 280 pharmaceutical products, primarily within the US.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.