Azitra has received the US Food and Drug Administration (FDA) fast track designation to its product ATR-04 for the treatment of skin rash from Epidermal Growth Factor Receptor (EGFR) inhibitors.
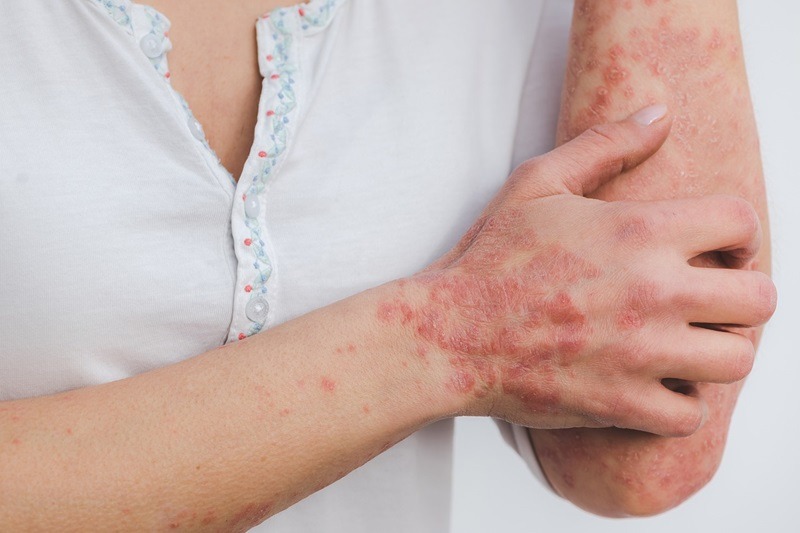
ATR-04 is being developed to address EGFRi-associated skin rash. Credit: Eszter Miller from Pixabay.
Subscribe to our email newsletter
This topical therapy is aimed at treating moderate to severe skin rash.
Azitra CEO Francisco Salva said: “Many cancer patients receive EGFR inhibitors, which often have significant side effects, resulting in rashes that require off-label treatment with antibiotics, steroids or other medications, or discontinuation of EGFRi therapy.
“The skin toxicity creates a high burden for these cancer patients, with a profound impact on their quality of life. We look forward to potentially accelerating the development of ATR-04 to treat this condition.”
The fast track programme is designed to expedite the development and review of new therapies that target serious conditions and fill unmet medical needs.
Benefits of this designation include more frequent FDA interactions and the possibility of accelerated approval.
ATR-04 is a live biotherapeutic product engineered to enhance safety by removing an antibiotic resistance gene and controlling growth through auxotrophy.
It is being developed to address EGFRi-associated skin rash, a condition that affects around 150,000 patients in the US and represents a market opportunity exceeding $1bn.
Azitra is preparing to launch a randomised, multicentre, controlled Phase I/II clinical trial of ATR-04 by the end of 2024.
This trial will focus on patients experiencing dermal toxicity due to EGFR inhibitors.
The company has also developed a platform that has a microbial library with approximately 1,500 unique bacterial strains.
This platform leverages artificial intelligence and machine learning to screen for therapeutic characteristics and drug-like molecules.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.

