Boehringer Ingelheim has received conditional marketing authorisation from the European Commission (EC) for its new humanised selective antibody, spesolimab, to treat adult patients with generalised pustular psoriasis (GPP) flares.
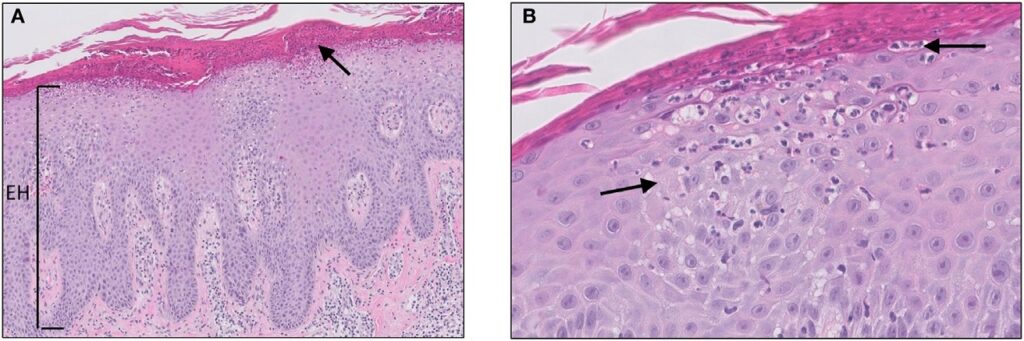
Micrograph of psoriasis vulgaris. Credit: Jenny Giang, Marc A. J. Seelen, Martijn B. A. van Doorn, Robert Rissmann,Errol P. Prens and Jeffrey Damman/ commons.wikimedia.org.
Subscribe to our email newsletter
Spesolimab has been developed to block the interleukin-36 receptor (IL-36R) activation.
IL-36R is a signalling pathway in the immune system that is involved in the pathogenesis of many autoinflammatory diseases, including GPP.
The new humanised antibody is currently being evaluated to prevent GPP flares as well as to treat other neutrophilic skin diseases.
Boehringer Ingelheim managing directors board member Carinne Brouillon said: “We are delighted to be able to bring this much needed treatment to patients with GPP, whose options were incredibly limited until this year.
“This approval marks another significant milestone in the continued development of spesolimab in neutrophilic skin diseases which we are investigating in further clinical trials.
“Today’s news marks the first of what we hope will be a number of new treatment options from our accelerated late-stage portfolio with the potential to transform the lives of people for generations to come.”
The conditional marketing authorisation is based on the data obtained from the pivotal EFFISAYIL 1 Phase II clinical trial, which was conducted in patients with GPP flare.
In the 12-week trial, the participants were treated with spesolimab or placebo and most of them at the outset of the trial had experienced a moderate or severe pustulation of the skin.
The trial demonstrated that 54% of the spesolimab-treated patients were free of pustules after one week of receiving a single dose compared with placebo.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.

