The China National Medical Products Administration (NMPA) has approved the supplemental New Drug Application (sNDA) for JW Therapeutics’ relmacabtagene autoleucel injection to treat relapsed or refractory follicular lymphoma.
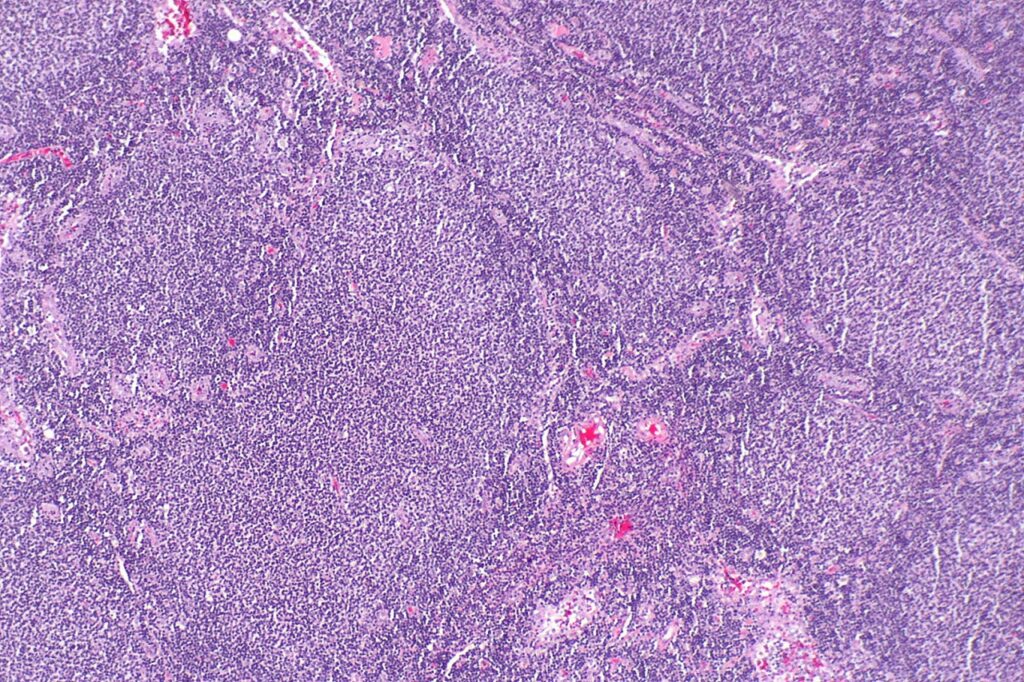
Micrograph showing a small B-cell lymphoma compatible with follicular lymphoma. Credit: Nephron / commons.wikimedia.org.
Subscribe to our email newsletter
The injection (abbreviated as relma-cel ) has been approved to treat adult patients with follicular lymphoma that relapses or is refractory in 24 months of second-line or above systemic treatment (r/r FL).
Developed by JW Therapeutics, relma-cel is an autologous anti-CD19 CAR-T cell immunotherapy product which has been developed based on Juno Therapeutics’ CAR-T cell process platform.
The regulatory approval marks relma-cel as the first cell immunotherapy product approved in China to treat r/r FL patients and the second approved indication after its launch in September last year.
It is based on the six months clinical data obtained from the cohort B of a multi-centre, single-arm pivotal RELIANCE study conducted on Carteyva in relapsed or refractory B cell non-Hodgkin lymphoma adult patients in China.
The findings demonstrated that Carteyva showed 92.58% overall response rate (ORR), 77.78% complete response rate (CRR) at month six, and controllable CAR-T associated toxicities in r/r FL patients.
JW Therapeutics co-founder, chairman and CEO James Li said: “Thanks to the patients and investigators who contributed to the clinical studies of Carteyva, and thanks to the regulators for the recognition of Carteyva.
“We are pleased with the second approved indication, which provides a new and breakthrough treatment option for r/r FL patients.
“JW Therapeutics is committed to maximising the value of Carteyva, continuously advancing technology innovation and pipeline development, and improving the accessibility of cell immunotherapy products.”
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.

