The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval of Bristol Myers Squibb’s Reblozyl (luspatercept) for adult patients with anemia associated with non‑transfusion-dependent (NTD) beta thalassemia.
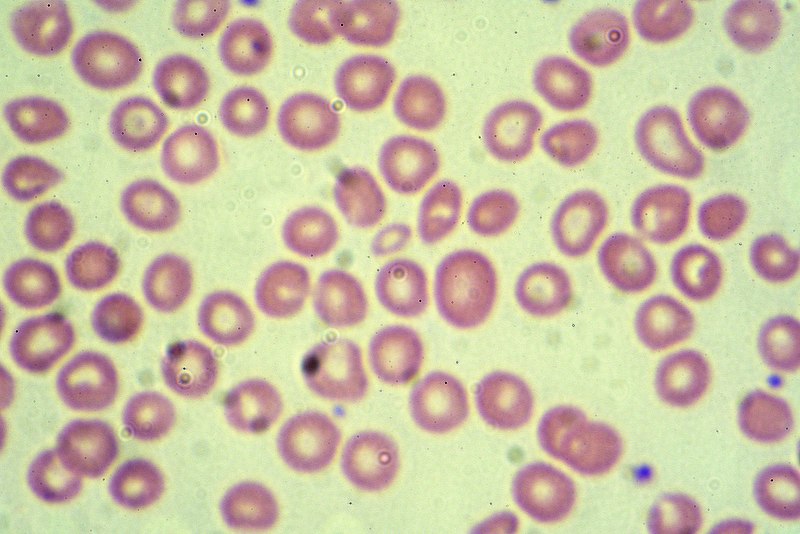
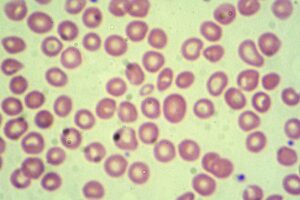
A giemsa stained blood smear from a person with beta thalassemia. Credit: Dr Graham Beards / commons.wikimedia.org.
Subscribe to our email newsletter
The European Commission (EC) will now review the recommendation. On receiving approval, this would represent the third authorised indication for the therapy in the EU.
Approved in the US, the EU, China and Canada, Reblozyl is an erythroid maturation agent and will address anemia-associated, transfusion-dependent beta thalassemia and transfusion-dependent lower-risk myelodysplastic syndromes.
The CHMP’s positive opinion for the therapy has been granted based on results obtained from the pivotal Phase II BEYOND study.
This trial assessed the efficacy and safety of Reblozyl versus placebo in 145 adult subjects with NTD beta thalassemia.
Bristol Myers Squibb Hematology Development senior vice-president Noah Berkowitz said: “Beta thalassemia is an inherited blood disorder that puts patients at significant risk for long-term clinical complications that can impair their quality of life, regardless of whether they require regular blood transfusions.
“Results from the BEYOND study showed Reblozyl improved anemia associated with non-transfusion-dependent beta thalassemia by sustaining hemoglobin increases in 77% of patients regardless of their baseline hemoglobin status.
“The potential approval of Reblozyl for patients with non-transfusion-dependent beta thalassemia represents an important development in the EU where several countries present with a high prevalence and where more people are impacted by the disease.”
The final decision from the EC is expected within 67 days of receipt of the CHMP opinion and will be applicable to all EU member states, Iceland, Norway and Liechtenstein.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.