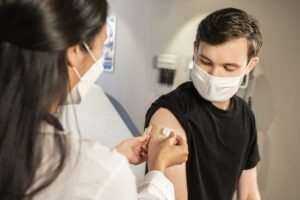
The US Food and Drug Administration (FDA) has expanded the emergency use authorisation (EUA) for Pfizer and BioNTech’s Covid-19 vaccine to include adolescents aged 12 to 15 years.
FDA approves emergency use of Pfizer-BioNTech Covid-19 vaccine in adolescents. Credit: Pete Linforth from Pixabay.
Subscribe to our email newsletter
The US Food and Drug Administration (FDA) has expanded the emergency use authorisation (EUA) for Pfizer and BioNTech’s Covid-19 vaccine to include adolescents aged 12 to 15 years.
With FDA authorisation, the vaccine is claimed to be the first product authorised for this age group in the country.
The authorisation is based on data from the Phase III clinical trial from a total of 2,260 subjects aged 12 to 15 years in the US.
All participants will be followed-up for long-term protection and safety for two years after receiving their second dose.
This trial showed 100% efficacy in subjects with or without prior SARS-CoV-2 infection and robust antibody responses.
Upon receipt of the second dose of the vaccine, the trial subjects will be observed for long-term protection and safety for two years.
Pfizer chairman and CEO Albert Bourla said: “Today’s expansion of our EUA represents a significant step forward in helping the U.S. government broaden its vaccination program and help protect adolescents before the start of the next school year.
“We are grateful to all of our clinical trial volunteers and their families, whose courage helped make this milestone possible. Together, we hope to help bring a sense of normalcy back to young people across the country and around the world.”
Additionally, a paediatric trial evaluating the efficacy and safety of the Covid-19 vaccine in children aged six months to 11 years is underway.
Based on BioNTech proprietary mRNA technology, the Covid-19 vaccine is developed by both BioNTech and Pfizer.
BioNTech holds the marketing authorisation in the European Union as well as emergency use authorisations (EUA) or similar approvals in the US, along with Pfizer, the UK, Canada and various other countries.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.