A global health care company Fresenius Kabi has announced the availability of Vasopressin injection, USP, in the US for treating adults with vasodilatory shock.
Subscribe to our email newsletter
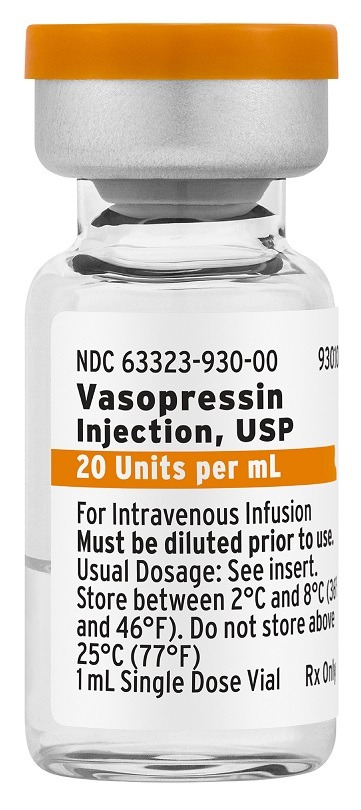
Vasopressin Injection is a generic equivalent to Par Pharmaceutical’s Vasostrict and is available in a single dose vial of 20 units per 1 mL.
Fresenius has invested nearly $1bn for the manufacture as well as distribution of Vasopressin to serve hospitals and health systems in the US.
Fresenius Kabi president and CEO John Ducker said: “Fresenius Kabi is pleased to offer Vasopressin for our US customers further demonstrating our continued commitment to providing high-quality, cost-saving treatment options to clinicians and the patients they serve.”
Adults with vasodilatory shock who remain hypotensive inspite of taking catecholamines and fluids benefit from using Vasopressin injection, which is indicated for increasing blood pressure.
Bradycardia, tachyarrhythmias, decreased cardiac output, ischemia, and hyponatremia are some of the adverse reactions on using Vasopressin.
Patients may also suffer from reversible diabetes insipidus after stopping treatment with vasopressin.
Co-administration of drugs causing SIADH or ganglionic blockers may increase the pressor response while co-administration of drugs causing diabetes insipidus may decrease the pressor response.
Vasopressin may induce uterine contractions when used in pregnancy and can also worsen cardiac function by decreasing cardiac index.
Furthermore, the company stated that the safety and effectiveness of Vasopressin have not been determined in pediatric use and no safety issues have been identified in geriatric (older patients) use.
In addition to injectable medicines, Fresenius is engaged in developing, biosimilars, and technologies for transfusion, infusion, and clinical nutrition.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.

