Jazz Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) approved Xywav (calcium, magnesium, potassium, and sodium oxybates) oral solution on July 21, 2020 for the treatment of cataplexy or excessive daytime sleepiness (EDS) in patients 7 years of age and older with narcolepsy.
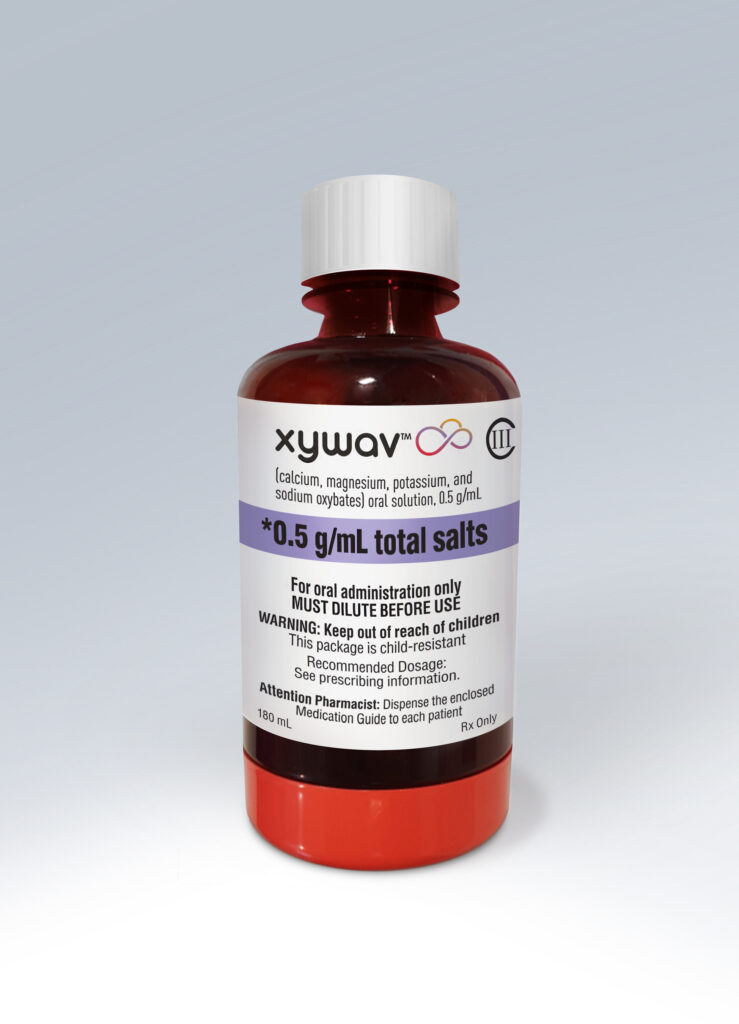
Jazz Pharmaceuticals gets US FDA approval of Xywav for cataplexy associated with narcolepsy. (Credit: PRNewsfoto/Jazz Pharmaceuticals plc)
Subscribe to our email newsletter
Xywav is an oxybate product with a unique composition of cations resulting in 92 percent less sodium – or approximately 1,000 to 1,500 mg/night – than sodium oxybate at the recommended dosage range of 6 to 9 grams.
The company plans to launch Xywav by the end of the year following Risk Evaluation and Mitigation Strategy (REMS) implementation. Jazz is committed to ensuring access to our medicines and will work to secure the broadest access possible for appropriate patients.
The FDA approval of Xywav is based on a global Phase 3 double-blind, placebo-controlled, randomized-withdrawal, multicenter study that demonstrated the efficacy and safety of Xywav in the treatment of cataplexy and EDS in patients with narcolepsy. In the study, which enrolled 201 patients, Xywav demonstrated highly statistically significant differences (p<0.0001) in weekly number of cataplexy attacks and Epworth Sleepiness Scale scores compared to placebo.
Multiple Xywav dosing options are available for adult and pediatric patients. Prescribers can titrate Xywav into unequal doses taken over the course of the night. When patients start Xywav after sodium oxybate, Xywav treatment is initiated at the same dose and regimen as sodium oxybate (gram for gram) and titrated as needed based on efficacy and tolerability.
“Based on the efficacy demonstrated in the clinical program, the approval of Xywav is important for people living with cataplexy or EDS associated with narcolepsy. Xywav makes it possible for patients to have a lower-sodium oxybate treatment option. This may help patients taking sodium oxybate better align with daily sodium intake recommendations including those by the American Heart Association,” said Richard K. Bogan, MD, FCCP, FAASM, associate clinical professor at the University of South Carolina School of Medicine, a medical officer at SleepMed in Columbia, SC and lead investigator of the Phase 3 study. “The average American consumes too much sodium.5 Excess sodium intake has been linked with increases in blood pressure, hypertension, stroke, and other cardiovascular disease.”
Sodium oxybate carries warnings about its high sodium content,10 and was previously the only product approved to treat both cataplexy and EDS in patients with narcolepsy 7 years of age and older11 and designated as a standard of care for the treatment of cataplexy and EDS by the American Academy of Sleep Medicine. With the goal of establishing a new standard of care, Xywav was developed to provide people with narcolepsy an oxybate therapy with lower sodium, and does not carry warnings about sodium content.
Xywav has a Boxed Warning as a central nervous system depressant, and for its potential for abuse and misuse. Because of the risks of CNS depression and abuse and misuse, Xywav is available only through a restricted program under a REMS called the Xywav and Xyrem REMS Program. Most common adverse reactions in adults (≥5%) were headache, nausea, dizziness, decreased appetite, parasomnia, diarrhea, hyperhidrosis, anxiety and vomiting.
“We have been working for nearly a decade to develop Xywav, a unique oxybate product with a significant reduction in sodium. We are proud to advance the science behind our sleep research program in order to continue making a difference for people living with narcolepsy,” said Bruce Cozadd, chairman and CEO of Jazz Pharmaceuticals. “Jazz is committed to addressing unmet needs in sleep medicine, which includes our innovative and long-standing oxybate program.”
Narcolepsy is a chronic neurologic condition with no cure and the illness burden can have a far-reaching impact on a patient’s health over time. As an established leader in sleep medicine, Jazz is commited to raising awareness about narcolepsy and helping patients find strategies to manage this sleep disorder.
“Many people with narcolepsy can go years before being properly diagnosed and this can have a significant impact to their everyday life,” said Julie Flygare, president and CEO of Project Sleep. “Narcolepsy is a life-long condition so it is important to have new options to help treat EDS and cataplexy.”
The U.S. Drug Enforcement Agency (DEA) has designated Xywav as a Schedule III medicine. The DEA defines Schedule III drugs, substances, or chemicals as drugs with a moderate to low potential for physical and psychological dependence.
Source: Company Press Release
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.