Omeros has received orphan drug designation from the US Food and Drug Administration (FDA) for OMS906 to treat paroxysmal nocturnal hemoglobinuria (PNH).
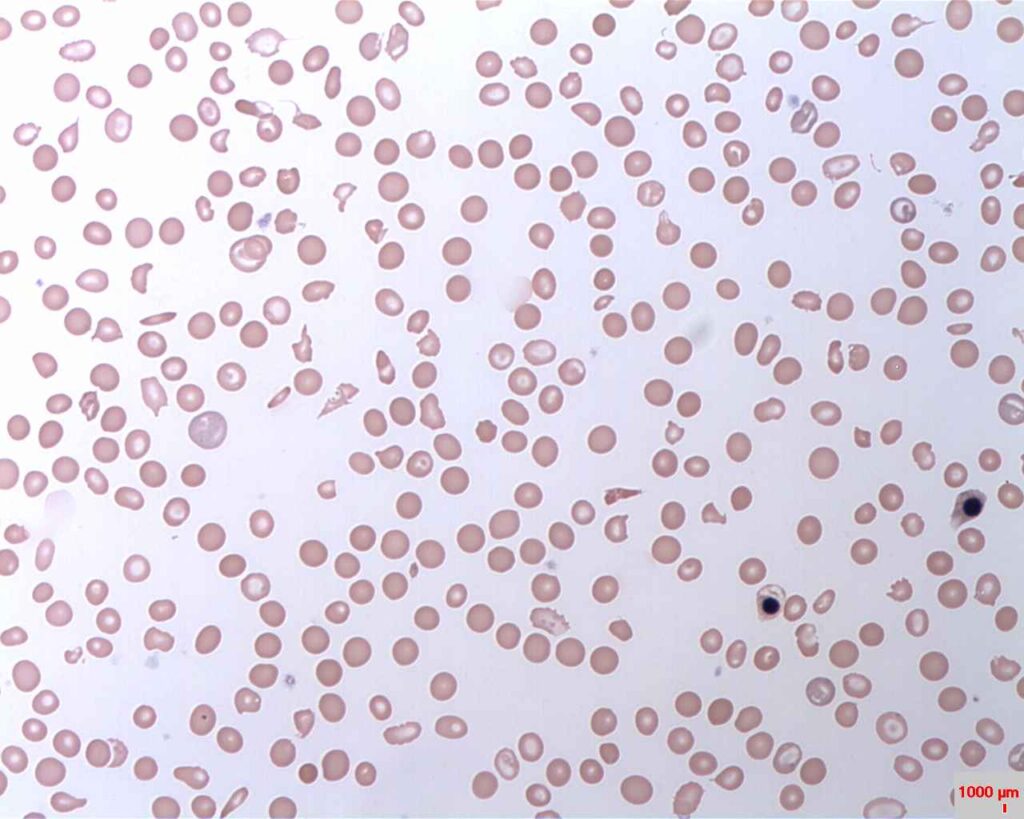
PNH is characterised by destruction in red blood cell, blood clots and impaired bone marrow function. Credit: Prof. Osaro Erhabor/ commons.wikimedia.org.
Subscribe to our email newsletter
The investigational human monoclonal antibody, OMS906 targets mannan-binding lectin-associated serine protease-3 (MASP-3), the main activator of the alternative pathway of the complement system.
MASP-3 converts pro-complement factor D (pro-CFD) to complement factor D and is believed to be the premier target in the alternative pathway.
Recently, the company completed a Phase I trial of the monoclonal antibody in healthy subjects.
The finding from the Phase I trial demonstrated that the antibody has potential to provide a favourable safety profile and better convenient dosing comparedwith other drugs in the market or under development to treat PNH.
Depending on the data, OMS906 is expected to be given monthly once to quarterly once intravenously or subcutaneously.
Omeros chairman and CEO Gregory Demopulos said: “We are excited to begin clinical studies to demonstrate the efficacy of OMS906 in alternative pathway-related disorders. Based on our preclinical and Phase 1 trial data, we expect that OMS906 will perform well.
“With the potential advantages of MASP-3 inhibition and OMS906 – decreased infection risk, better dosing profile, and the ability to avoid ‘breakthrough’ disease seen with agents targeting acute phase reactants – we believe that OMS906 could become first-line therapy in alternative pathway disorders.”
This summer, the company expects to commence enrolling subjects for a clinical study to evaluate OMS906 in PNH patients with unsatisfactory response to the C5 inhibitor ravulizumab.
It is commencing Phase Ib clinical programmes on OMS906 in paroxysmal nocturnal hemoglobinuria (PNH) and complement 3 glomerulopathy (C3G), with efficacy data expected by early next year.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.

