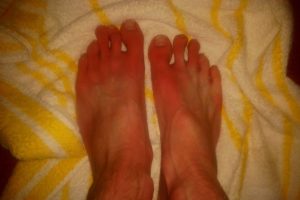
AlgoTherapeutix announces orphan drug designation granted by fda for atx01 in erythromelalgia
This designation is given to medications with the potential to treat rare conditions — those that affect fewer than 200,000 people in the U.S. Benefits include exemption from
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.