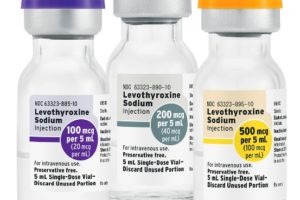
Fresenius Kabi introduces Levothyroxine Sodium Injection Solution
Fresenius Kabi Levothyroxine Sodium Injection is the first-and-only FDA-approved solution formulation for this medicine available in the U.S. Fresenius Kabi also sells Levothyroxine Sodium for Injection as a
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.