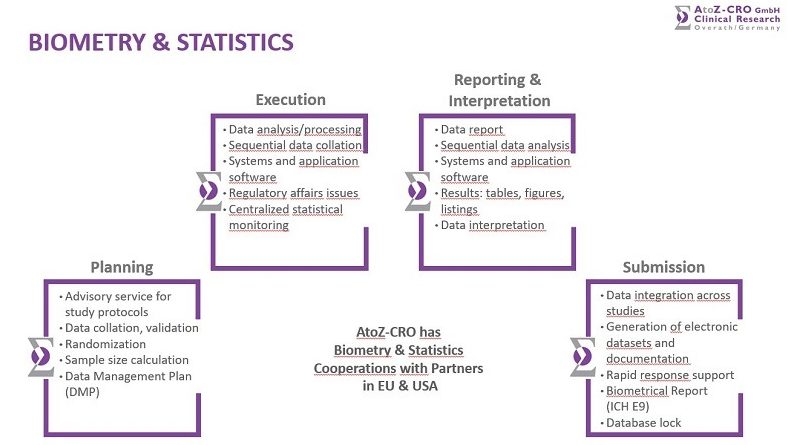
Our partners in the EU and in the USA provide clients with integrated, global, therapeutically aligned, end-to-end biostatistics services through all stages of the clinical trial development, i.e. planning, execution, reporting & interpretation and submission.
Our partners are constantly looking for optimal statistical methods in both the design of clinical trials as well as their conduct and statistical analysis and reporting. Also, in the commercialization phase we are looking together with our partners for cost-effective but impact generating solutions.
The advantages are an improved drug development performance/timeline, a cost-effective and tailored trial design, an effective risk management (go/no go decisions) and a practical data-driven insight generation.