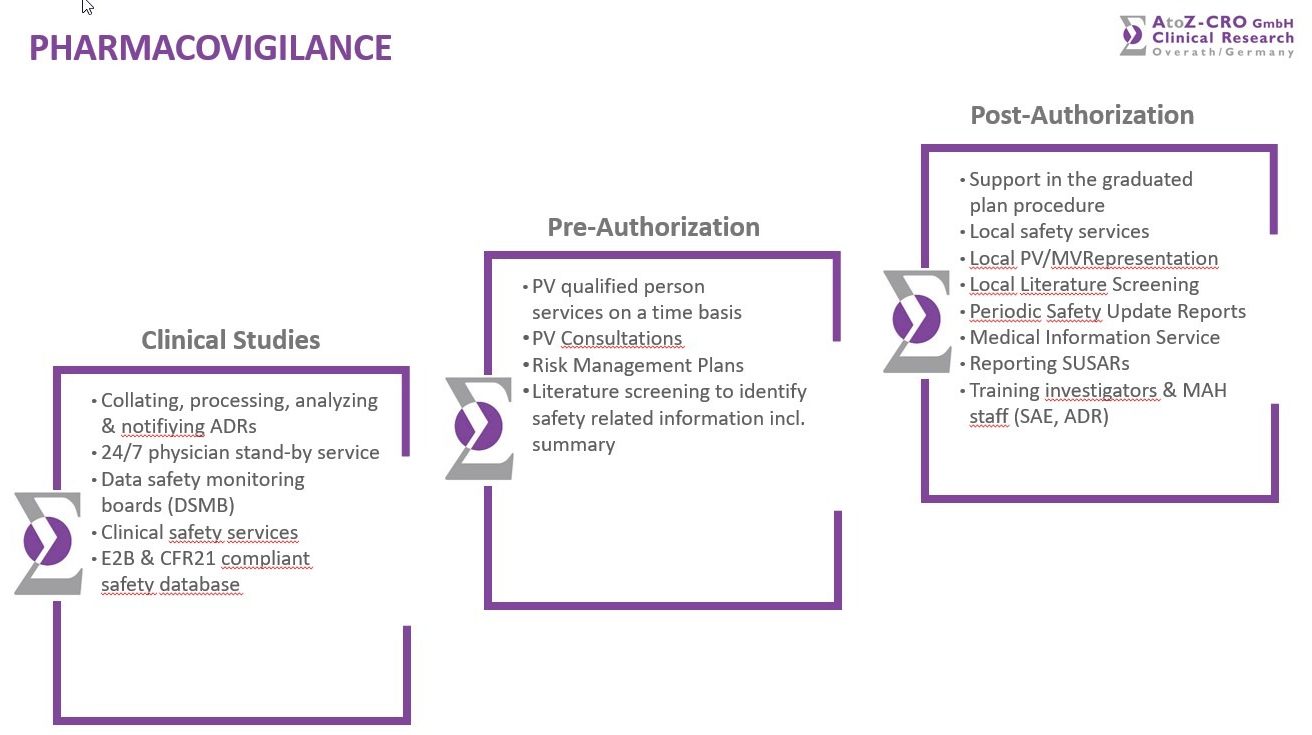
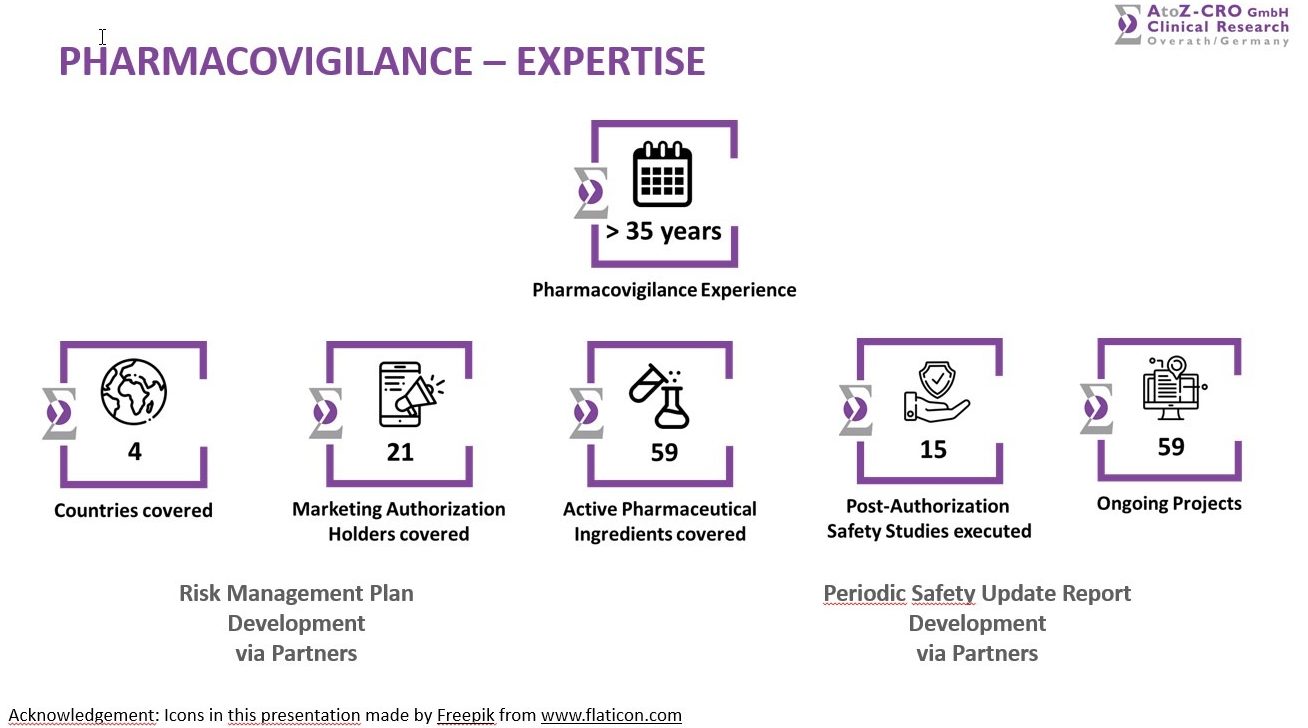
The collating, processing, analyzing and notifying of ADRs during clinical studies is key and one of AtoZ’ competences. The submission of SUSARs and ICSR to European Regulatory Authorities and CECs is done as well as the specialization as an EMA certified safety monitoring for E2B submissions of SUSARs and ICSRs. Medical, clinical and safety regulatory affairs in the EU and a 24-hour physician stand-by service is included as well. Two AtoZ’ employees are certified and empowered to take care of any issue regarding the Pharmacovigilance.