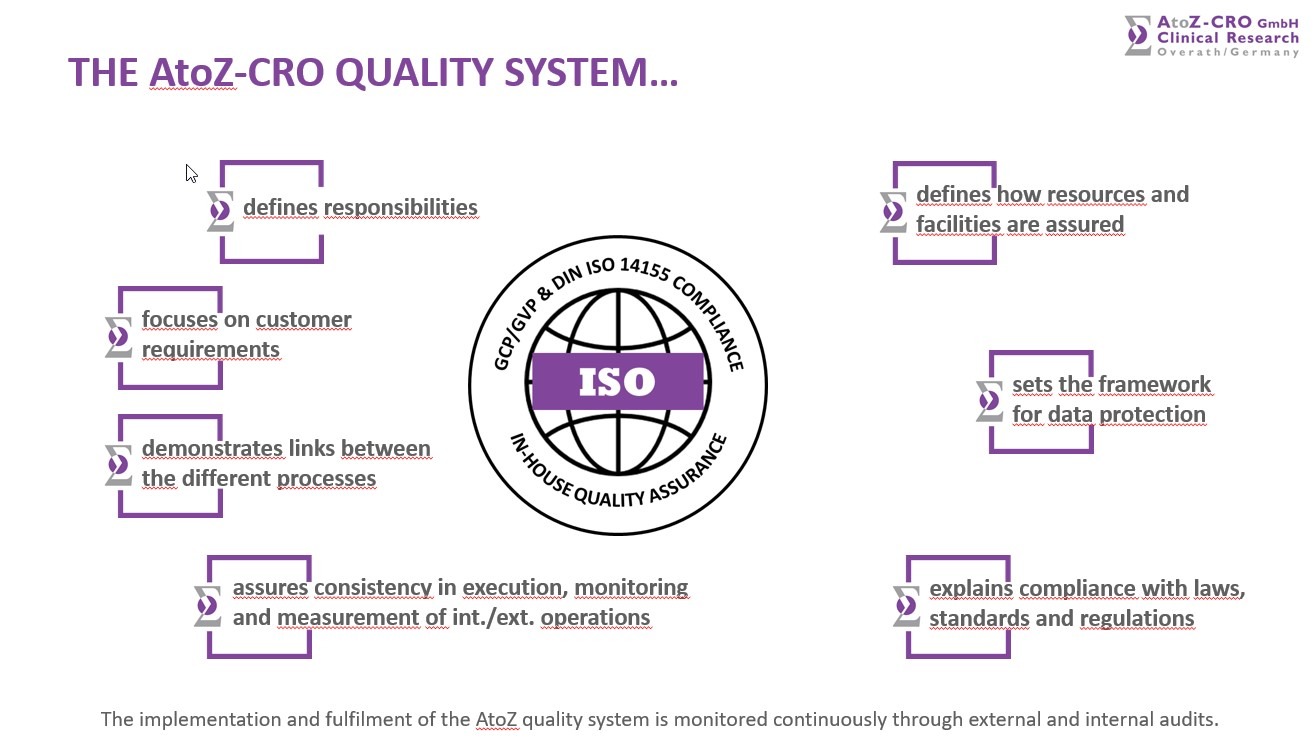
At AtoZ-CRO, the core business principle is to serve the customer with the highest flexibility and to fulfil customer requirements as specifically as possible. AtoZ continuously strives to provide services of the highest quality and greatest value with respect to legal compliance and to the satisfaction of the sponsor and authorities.
The company has its own quality management department. Independent audits of study documents (clinical study protocol, subject information sheet/informed consent form, case report form, clinical study report), investigator sites, database and Trial Master Files can be conducted as well as external supplier audits (including special service providers such as IVRS, ECG, clinical trial supply chain management) and internal system audits.