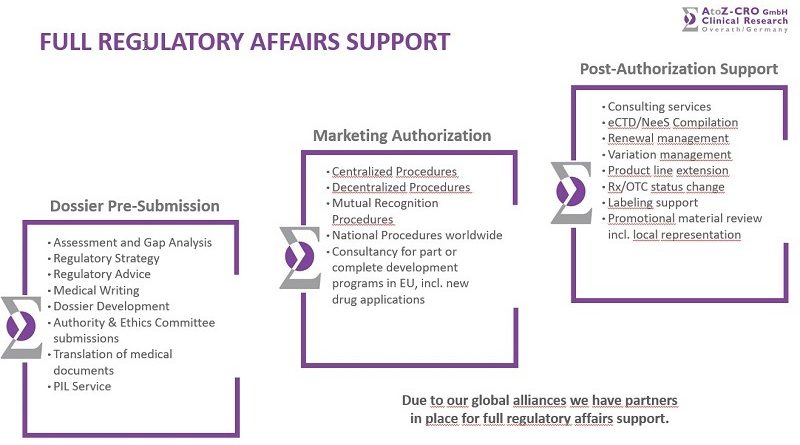
Due to our global alliances, we have partners in place for full regulatory affairs support.
Via our partners we are providing complete solutions for marketing medicines predominantly in the European Union, taking care of all pharmaceutical responsibilities and activities.
For new marketing authorisations, we develop custom-tailored regulatory strategies. Based on our long-time experience with many European regulatory agencies, we provide guidance during the procedure or even manage the entire procedure on behalf of the applicant.
We provide regulatory support throughout the lifecycle of your products. Our services are tailored to your needs and cover all aspects of lifecycle management.
For electronic submissions, we compile dossiers according to the current specifications developed by the ICH, the European Union and Switzerland.
Our clinical experts provide professional medical writing services. In order to guarantee professional and technically accurate translations, we operate our own network of native speakers with a university degree in pharmacy, medicine or similar life sciences.