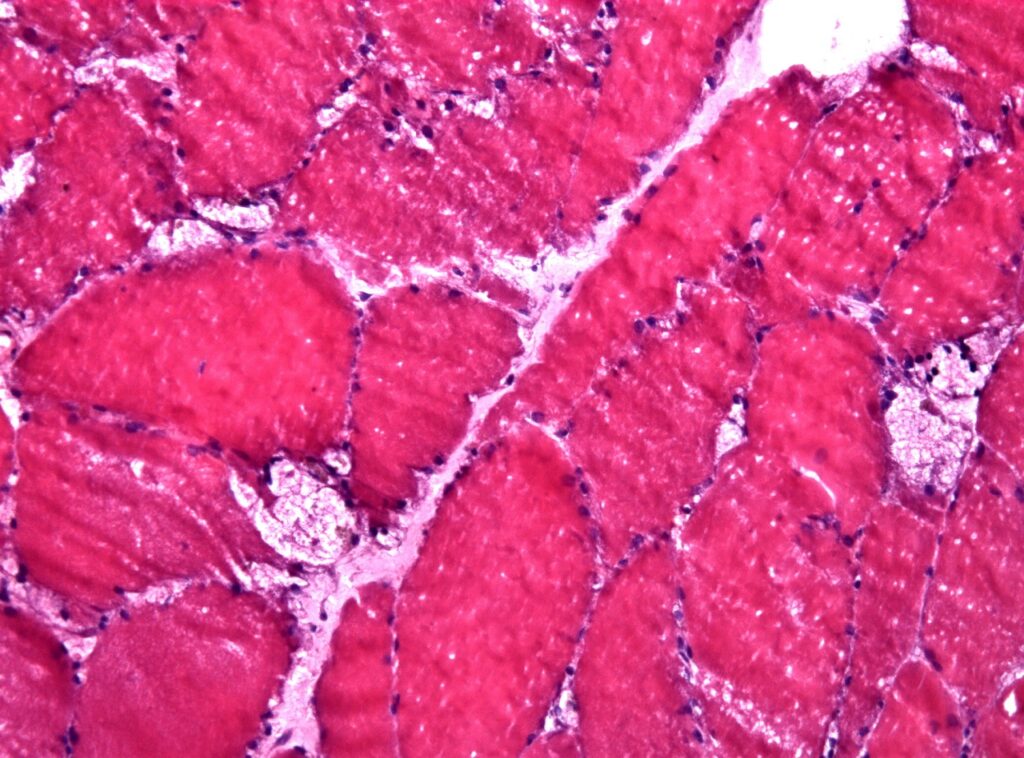
EMA grants PRIME designation to Rocket’s AAV-based gene therapy
The European Medicines Agency (EMA) has granted Priority Medicines (PRIME) designation to Rocket Pharmaceuticals’ adeno-associated virus (AAV)-based gene therapy, RP-A501, for the treatment of Danon Disease, a rare X-linked inherited disorder.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.