FDA approves ViiV Healthcare’s Apretude for HIV prevention
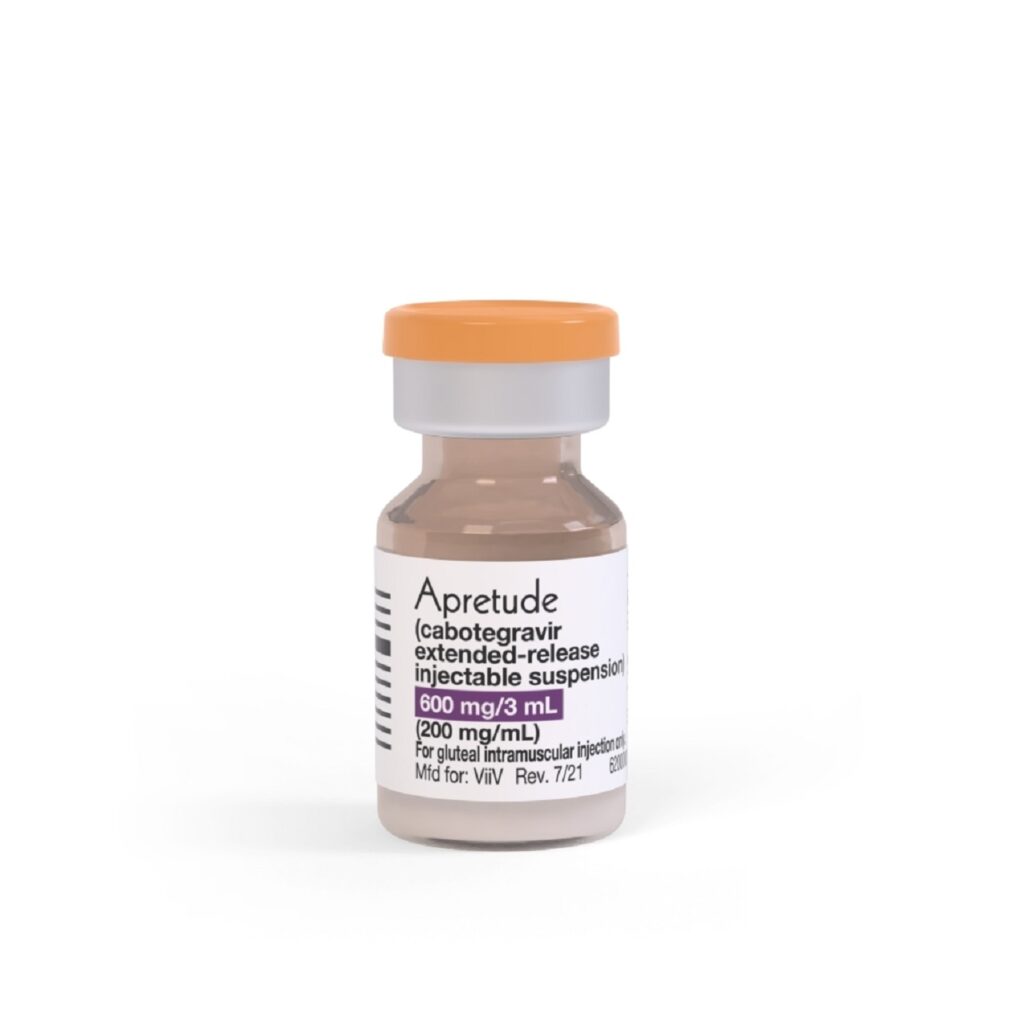
ViiV Healthcare has received the US Food and Drug Administration (FDA) approval for Apretude (cabotegravir extended-release injectable suspension) as an injectable pre-exposure prophylaxis (PrEP) option for HIV prevention.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.