Jazz Pharmaceuticals gets US FDA approval of Xywav for cataplexy associated with narcolepsy
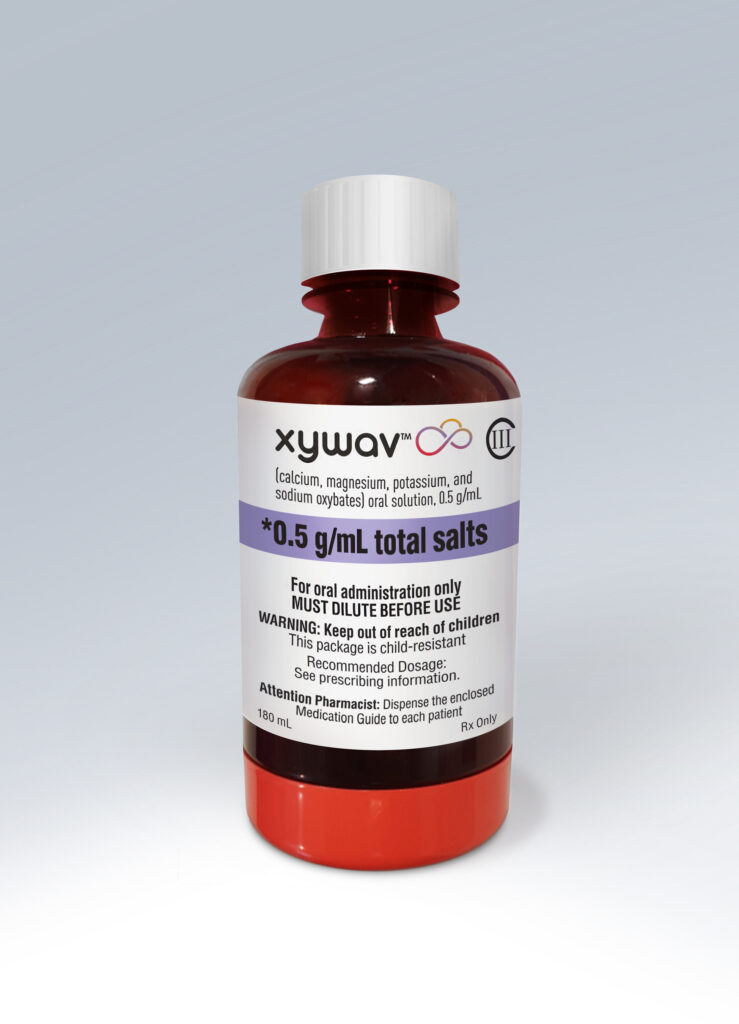
Jazz Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) approved Xywav (calcium, magnesium, potassium, and sodium oxybates) oral solution on July 21, 2020 for the treatment of cataplexy or excessive daytime sleepiness (EDS) in patients 7 years of age and older with narcolepsy.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.