June 27, 2022
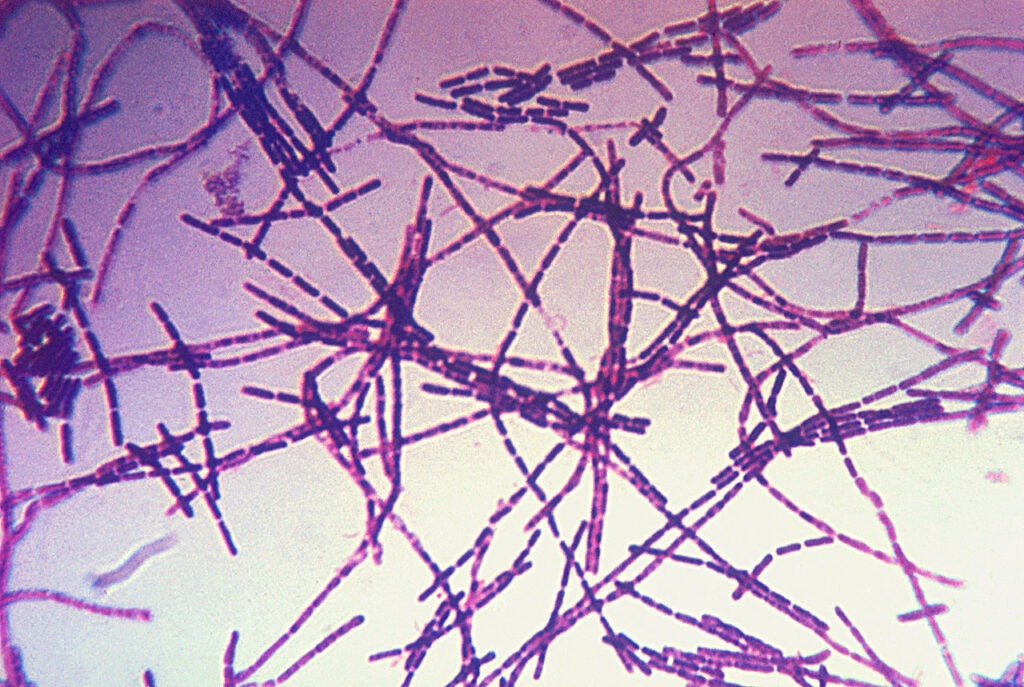
FDA accepts Emergent BioSolutions’ anthrax vaccine candidate for review
The vaccine candidate seeks to prevent anthrax in people after suspected or confirmed exposure to Bacillus anthracis. AV7909 is being assessed in adult persons between 18 and 65
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.