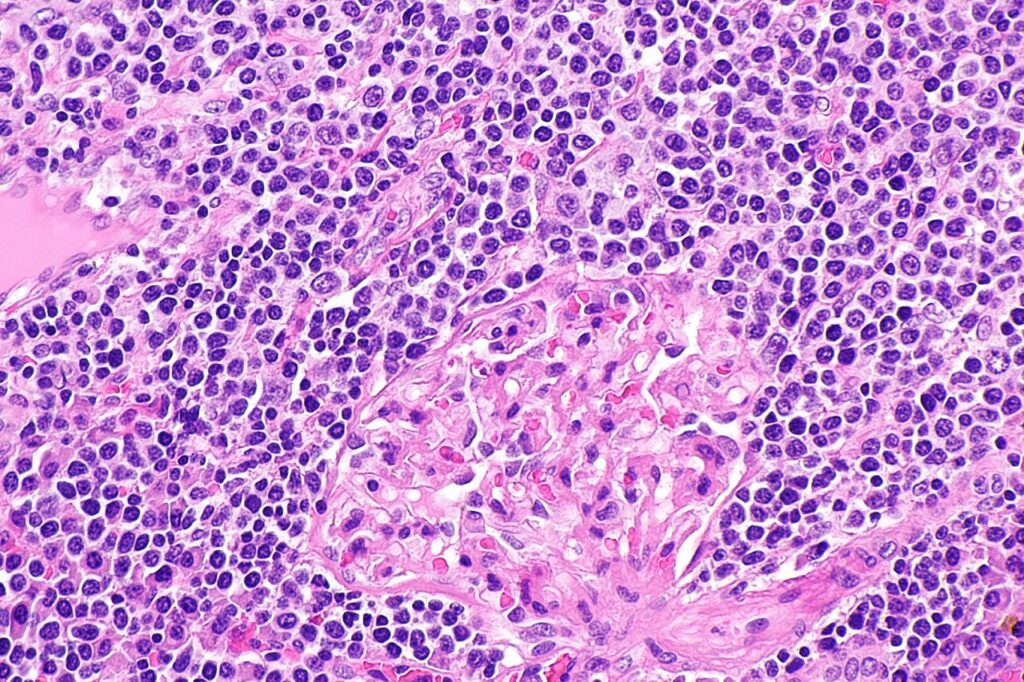
China’s NMPA approves InnoCare’s orelabrutinib to treat Marginal Zone Lymphoma
Orally available bruton’s tyrosine kinase (BTK) inhibitor, orelabrutinib irreversibly binds to BTK for inducing downstream kinase inactivation and cell death. It has been designed with a single ring
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.