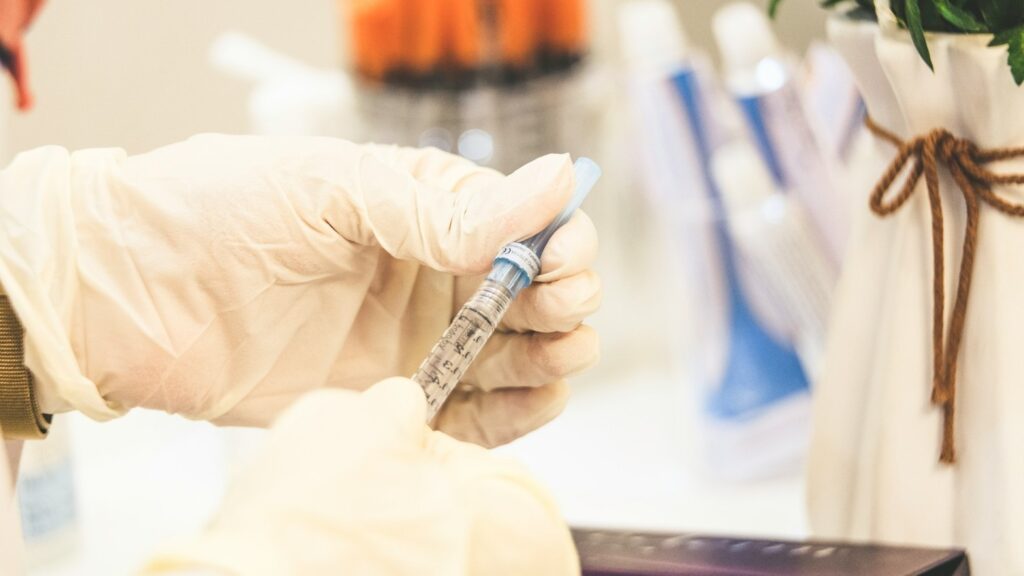
MITEM PHARMA acquires global rights to injectable medicine from Novartis
An injectable treatment, DESFERAL is intended to treat iron overload conditions, often arising from blood transfusions in patients with beta-thalassaemia and sickle-cell anaemia. Beta-thalassaemia and sickle cell anaemia
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.