Sandoz makes available biosimilar of Humira in US
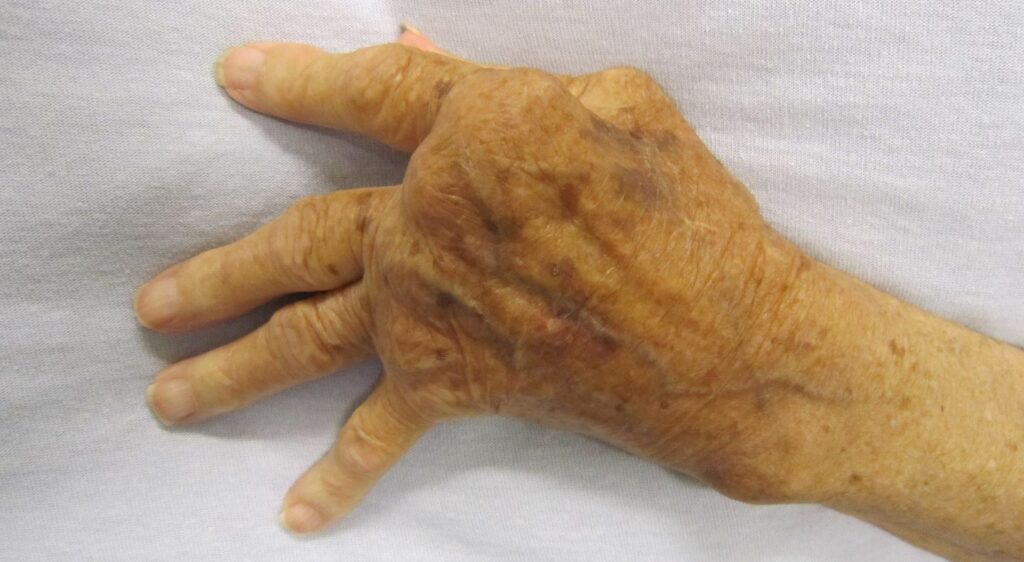
The treatment is indicated for usage in areas that are not covered by the reference medicine Humira’s regulatory exclusivity. The indications include inflammatory ailments namely juvenile idiopathic arthritis,
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.