The US Food and Drug Administration (FDA) has accepted the supplemental biologics license application (sBLA) for Sanofi and Regeneron’s Dupixent (dupilumab) for priority review in adults with prurigo nodularis.
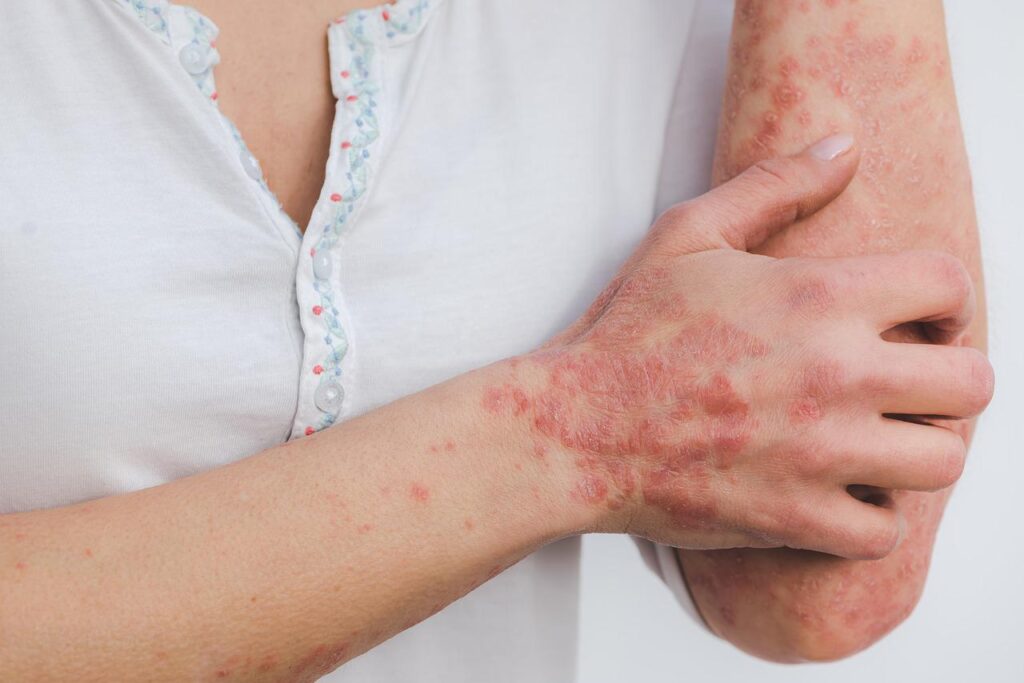
Prurigo nodularis is a chronic inflammatory skin disease that causes itch and skin lesions. Credit: Eszter Miller from Pixabay.
Subscribe to our email newsletter
The companies are seeking approval for the antibody to treat adult patients with prurigo nodularis, which is a chronic inflammatory skin disease that causes extreme itch and skin lesions.
The regulatory body is expected to take a decision by 30 September this year.
Dupixent is a fully human monoclonal antibody which has been designed to block the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways signalling.
It has also been approved to treat chronic rhinosinusitis with nasal polyposis, atopic dermatitis, asthma, and eosinophilic esophagitis in different age groups in many countries globally.
Currently, the antibody is approved across these indications in the US and for one or more indications in Japan, the European Union, as well as more than 60 countries.
The sBLA submitted to the FDA is supported by the data obtained from two Phase III clinical trials (PRIME2 and PRIME), which are assessing the safety and efficacy of the antibody in patients aged 18 years and above with uncontrolled prurigo nodularis.
This data showed that Dupixent significantly improved disease signs and symptoms, including reduction in itch and skin lesions compared to placebo.
The most common adverse event with the antibody was conjunctivitis.
Sanofi stated that the additional regulatory filings for the antibody are also planned this year.
Last month, the US FDA accepted the companies’ sBLA of Dupixent for priority review in adult and paediatric patients aged 12 years and above with eosinophilic esophagitis (EoE).
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.

