Gilead company Kite has received a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for its Yescarta (axicabtagene ciloleucel) to treat large B-cell lymphoma.
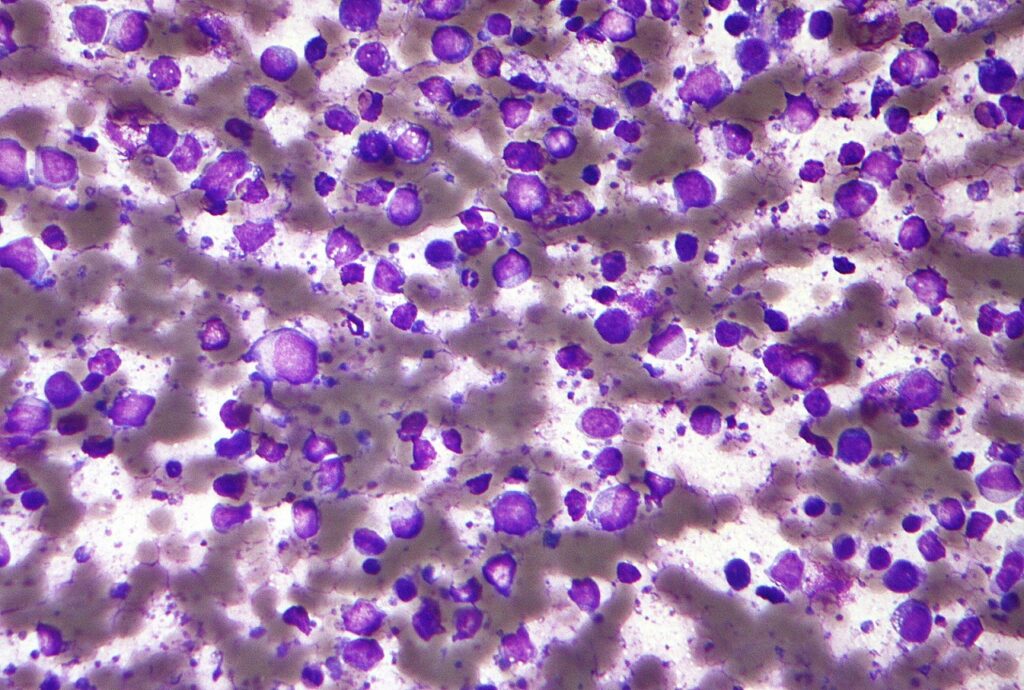
Micrograph of a diffuse large B cell lymphoma. Credit: Nephron / commons.wikimedia.org.
Subscribe to our email newsletter
Yescarta is a chimeric antigen receptor (CAR) T-cell therapy that is indicated to treat diffuse large B-cell lymphoma (DLBCL) and high-grade B-cell lymphoma (HGBL) in adult patients whose disease relapses within 12 months from completion of or is refractory to first line chemoimmunotherapy.
It will be the first CAR T-cell therapy approved to treat large B-cell lymphoma patients in Europe, if approved.
Kite CEO Christi Shaw said: “At Kite, we are committed to bringing the curative potential of cell therapy to the world and changing the way cancer is treated.
“Today’s positive CHMP opinion brings us a step closer to utilising cell therapy earlier in the treatment journey, potentially transforming the standard of care for the most common and aggressive form of non-Hodgkin lymphoma.”
The positive CHMP opinion is based on the primary results obtained from the Phase III ZUMA-7 study, which was conducted in 359 participants at 77 centres.
The global, randomised, multicentre, open-label, ongoing study was designed to assess the efficacy and safety of single-infusion of Yescarta compared to the current standard of care (SOC) for second-line therapy to treat relapsed or refractory LBCL adult patients within 12 months of first-line therapy.
Findings showed that the patients treated with Yescarta had improvement in event-free survival over the current SOC.
The therapy has also demonstrated 2.5 fold improvement in patients who were alive at two years without disease progression or need for additional cancer treatment compared to SOC.
The regulatory decision on the marketing authorisation for the therapy is expected in the coming months.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.

