Vanda Pharmaceuticals signed a research and development collaboration agreement with OliPass to jointly develop antisense oligonucleotide (ASO) therapeutics.
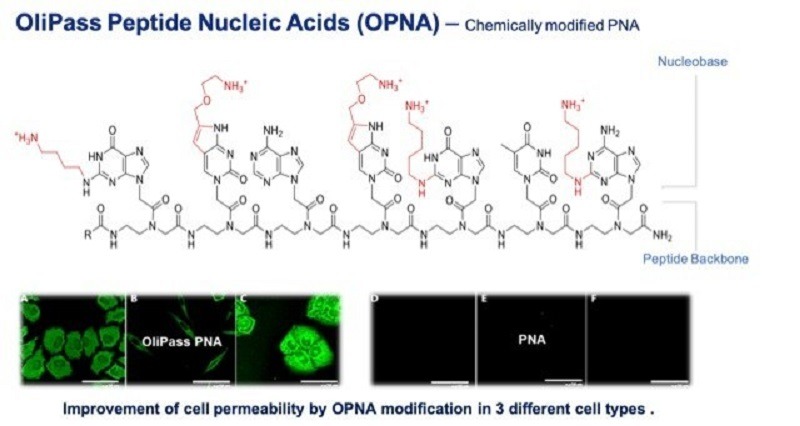
Olipass Peptide Nucleic Acids technology provides the delivery platform to enable the gene expression changes. Credit: Vanda Pharmaceuticals Inc./ PRNewswire.
Subscribe to our email newsletter
The collaboration will use the companies’ respective strengths to support the development of ASO-based precision medicine therapies and for creating compelling value opportunities for Vanda and OliPass.
It will focus on altering and editing gene expression using ASOs in disease states where the genes expression is either modified or the expressed genes’ sequence can be changed for treatment benefit.
Vanda stated that the Olipass Peptide Nucleic Acids (OPNA) technology offers the delivery platform to enable these gene expression modifications.
Vanda president, CEO and board chairman Mihael Polymeropoulos said: “We are excited to have a partner to enhance our antisense oligonucleotide program platform aimed at the treatment of disorders with well-understood genetic mechanisms and for which there is a high unmet medical need.
“Olipass’ delivery platform holds the promise of efficient delivery of our ASO’s in pursuit of the development of precision therapeutics.”
The company stated that it has already identified two ASO targets that have been validated in cell lines that model two undisclosed disease targets.
The disease targets include a rare orphan, while the other is applicable to a broad set of immuno-oncological conditions.
Vanda noted that its collaboration with OliPass to improve the existing ASOs with the latter’s OPNA chemistry is the next step to move the preclinical findings to in vivo and clinical testing.
OliPass CEO Dr. Shin Chung said: “Vanda shares a vision with us on the potential for anti-sense oligonucleotides to open up a broad range of therapeutic options to patients.
“We look forward to working together to enhance the therapeutic profile of these molecules and playing an integral role in bringing them into the clinical setting.”
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.

