Certa Therapeutics has received orphan drug designation from the US Food and Drug Administration (FDA) for its investigational therapy, FT011, to treat systemic sclerosis (scleroderma).
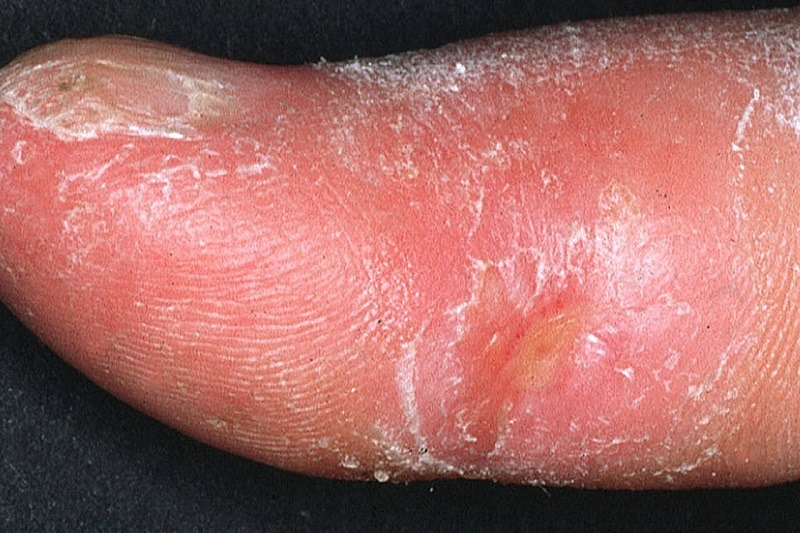
Clinical appearance of acrosclerotic piece-meal necrosis of the first digit in a patient with systemic sclerosis. Credit: Frank Breuckmann, Thilo Gambichler, Peter Altmeyer and Alexander Kreuter / commons.wikimedia.org.
Subscribe to our email newsletter
FT011 is a first-in-class oral therapy designed to treat chronic fibrosis in multiple organs. It is currently progressing through clinical development for the treatment of scleroderma.
It acts on a membrane GPCR receptor supported by a substantial body of evidence showcasing its efficacy in various fibrotic disease models.
Earlier, Certa reported positive top-line findings from a Phase II study, revealing that 60% of patients receiving FT011 400mg for 12 weeks experienced a clinically significant improvement.
In this trial, 20% of patients in the FT011 200mg experienced similar benefits, compared to just 10% in the placebo group.
In the pooled FT011 groups, three patients reached a maximum CRISS score of 1.0, indicating the highest likelihood of clinical improvement.
Certa Therapeutics CEO and founder professor Darren Kelly said: “We are very pleased that the FDA has granted orphan drug designation to FT011 which we believe highlights the urgent need for innovation and new therapeutic options for scleroderma patients.
“This designation represents an important milestone in the development of FT011, which has the potential to establish first-in-class clinical benefits by precisely targeting the root cause of fibrosis and offer treatment across multiple organs within these patients.”
Scleroderma is a severe autoimmune condition that can cause inflammation and fibrosis in the skin and various organs, such as the kidneys, lungs, and heart.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.

