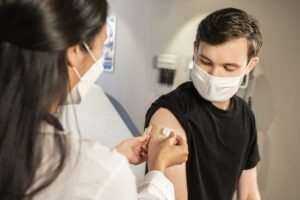
The US Food and Drug Administration (FDA) has approved ViiV Healthcare’s Dovato (dolutegravir/lamivudine) to treat HIV-1 infection in adolescents.
The FDA approval introduces a once-daily, two-drug regimen for individuals aged 12 years and above, weighing a minimum of 25kg. Credit: danilo.alvesd on Unsplash.
Subscribe to our email newsletter
This approval introduces a once-daily, oral, two-drug regimen for individuals aged 12 years and above, weighing a minimum of 25kg.
Dovato, which combines dolutegravir (50mg) with lamivudine (300mg), is designed for patients with no history of antiretroviral (ARV) treatment or for replacing the existing, stable ARV regimen in individuals who are virologically suppressed.
These patients should also not have failed treatment priorly and not have resistance to the individual Dovato components.
The FDA’s approval is based on the data from the Phase IIIb DANCE clinical trial of Dovato in treatment-naïve adolescents as well as evidence from adult trials, including GEMINI-1, GEMINI-2, and TANGO.
The single-arm, multicentre, open-label DANCE trial assessed the efficacy of Dovato as initial ARV treatment in adolescents with HIV-1 RNA levels ranging from 1,000 to 500,000 c/mL.
Proportion of subjects achieving HIV-1 RNA <50 c/mL at week 48 was the trial’s primary endpoint.
Trial data showed that 26 out of 30 subjects attained viral suppression at week 48.
Dovato’s safety and efficacy observed in adolescents were comparable to those seen in adult populations, with higher but not clinically significant exposures to the components of Dovato.
ViiV Healthcare North America head Lynn Baxter said: “This expanded indication for Dovato brings an oral, two-drug, single-tablet regimen to adolescents living with HIV, providing a complete HIV therapy with fewer ARV medicines – an important consideration for young people who will require lifelong treatment.
“As a leader in HIV, ViiV Healthcare is proud of our focused efforts to improve and expand care for children and adolescents and we remain committed to addressing the existing treatment gaps in these communities.”
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.