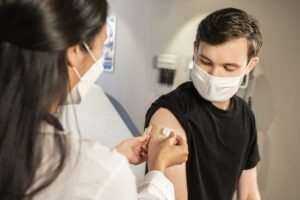
The European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended the granting of marketing authorisation for Moderna's mRESVIA (mRNA-1345) vaccine for adults aged 60 and above.
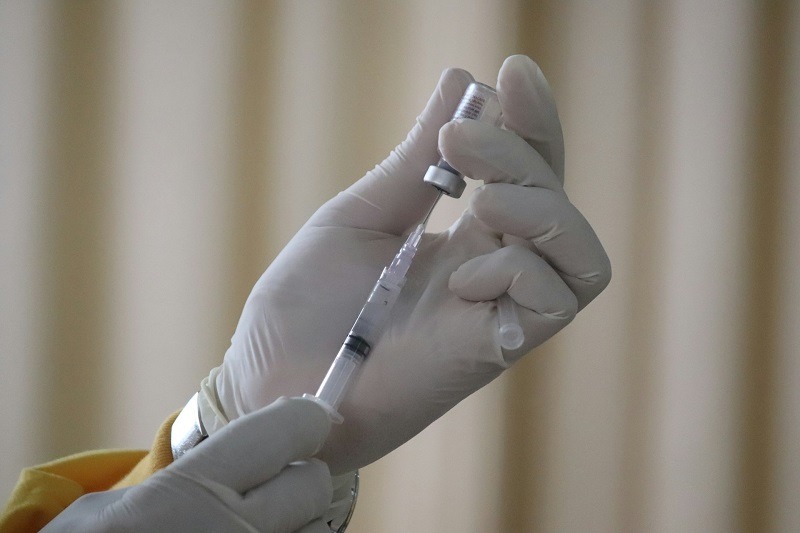
The vaccine employs the same lipid nanoparticle technology as Moderna's Covid-19 vaccines. Credit: Mufid Majnun on Unsplash.
Subscribe to our email newsletter
This endorsement is a significant step towards the authorisation of the vaccine in the European Union (EU) to prevent lower respiratory tract disease caused by the respiratory syncytial virus (RSV) infection.
mRESVIA is an mRNA vaccine targeting the F glycoprotein of RSV, which facilitates viral entry into host cells.
The vaccine employs the same lipid nanoparticle technology as Moderna’s Covid-19 vaccines.
The CHMP’s positive opinion is based on the Phase III ConquerRSV clinical trial, which involved approximately 37,000 participants across 22 countries.
The trial’s primary analysis, with a median follow-up of 3.7 months, demonstrated an 83.7% vaccine efficacy (VE) against RSV lower respiratory tract disease (LRTD).
A supplementary analysis with a median follow-up of 8.6 months showed sustained VE of 63.3% against RSV-LRTD, including cases with two or more symptoms.
Moderna CEO Stéphane Bancel said: “mRESVIA safeguards older adults against severe RSV outcomes and is uniquely offered in a pre-filled syringe to enhance ease of administration, which can save healthcare professionals time and reduce administrative errors.
“With mRESVIA, we continue to make significant strides in addressing global public health challenges posed by respiratory diseases, and we look forward to the decision on an EU-wide marketing authorisation from the European Commission.”
The European Commission is expected to follow the CHMP’s recommendation and grant marketing authorisation for mRESVIA.
In May this year, the US Food and Drug Administration (FDA) granted approval for the vaccine under a breakthrough therapy designation, marking Moderna’s second approved mRNA product.
Moderna has also submitted marketing authorisation applications for mRNA-1345 in various global markets.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.