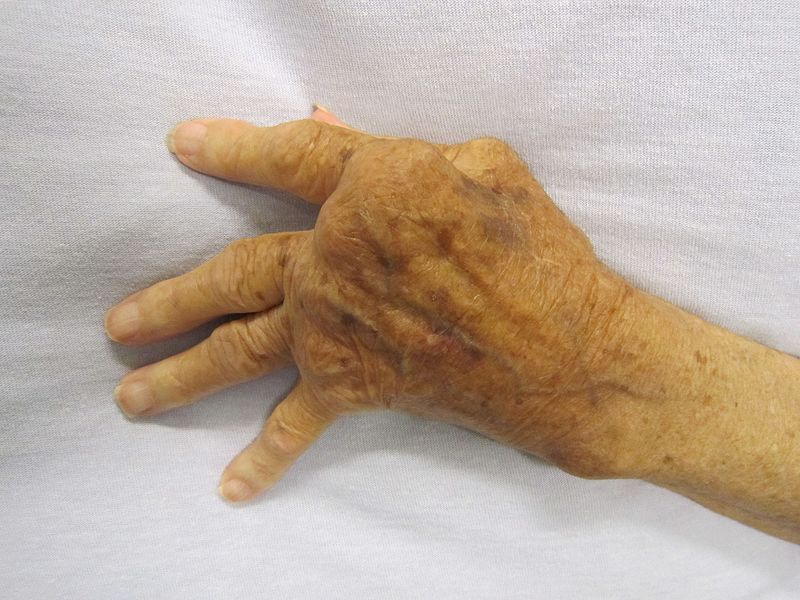
AbbVie receives FDA approval of RINVOQ for moderate to severe rheumatoid arthritis
AbbVie announced that the U.S. Food and Drug Administration (FDA) has approved RINVOQ (upadacitinib), a 15 mg, once-daily oral Janus kinase (JAK) inhibitor, for the treatment of adults with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response or intolerance to methotrexate (MTX-IR).
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.