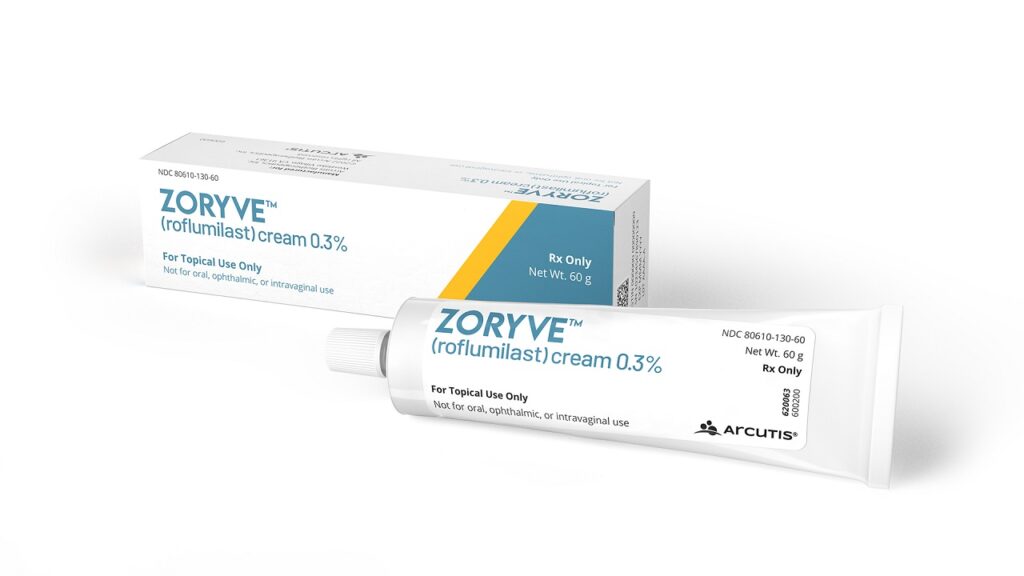
Health Canada approves Arcutis Biotherapeutics’ Zoryve for Plaque Psoriasis
The next generation topical phosphodiesterase-4 (PDE4) inhibitor Zoryve is also indicated to treat psoriasis in the intertriginous areas. It uses a drug delivery formulation, HydroARQ Technology, which creates
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.