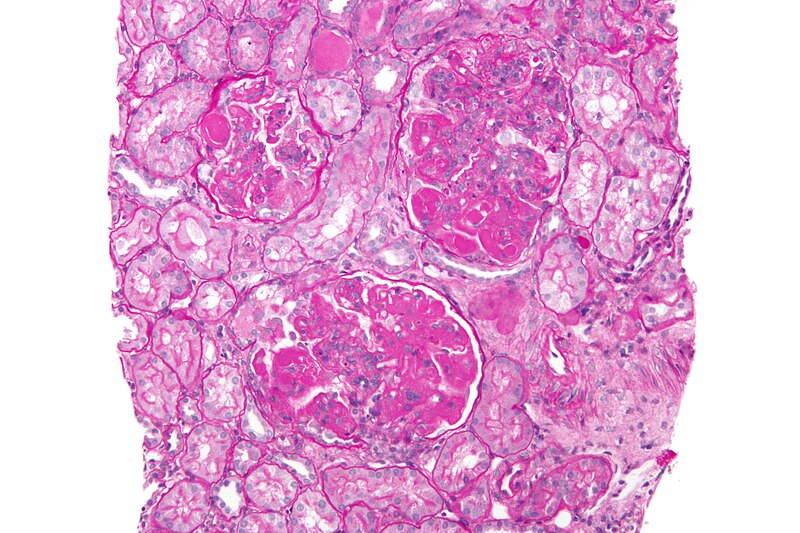
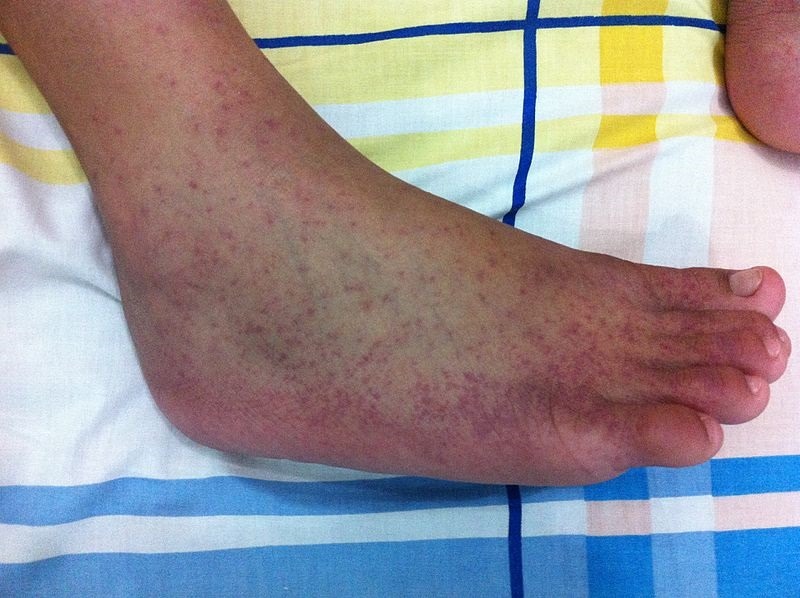
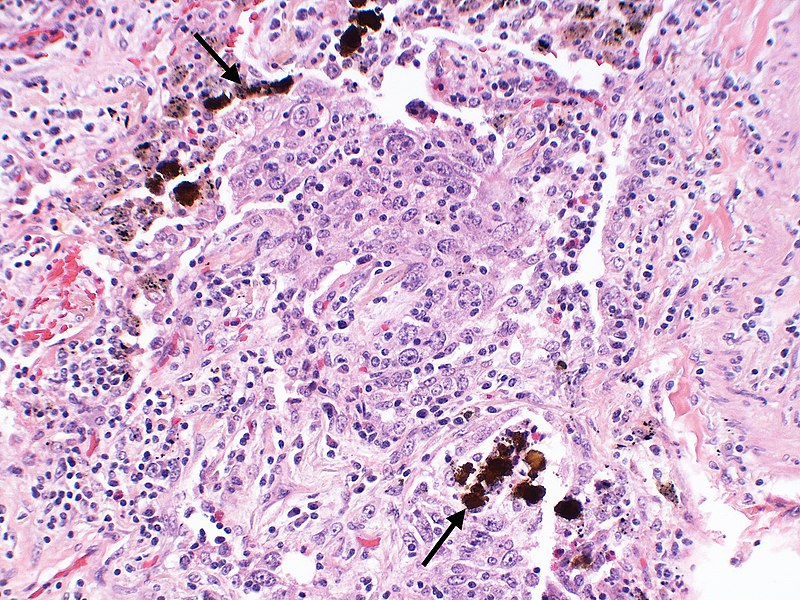
Otsuka Pharmaceutical submits NDA for voclosporin in Japan
The company filed the application to manufacture and sell the second-generation oral calcineurin inhibitor, voclosporin, in Japan. In January 2021, voclosporin along with a background immunosuppressive therapy regimen
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.