CanariaBio receives orphan drug designation for pancreatic cancer therapy
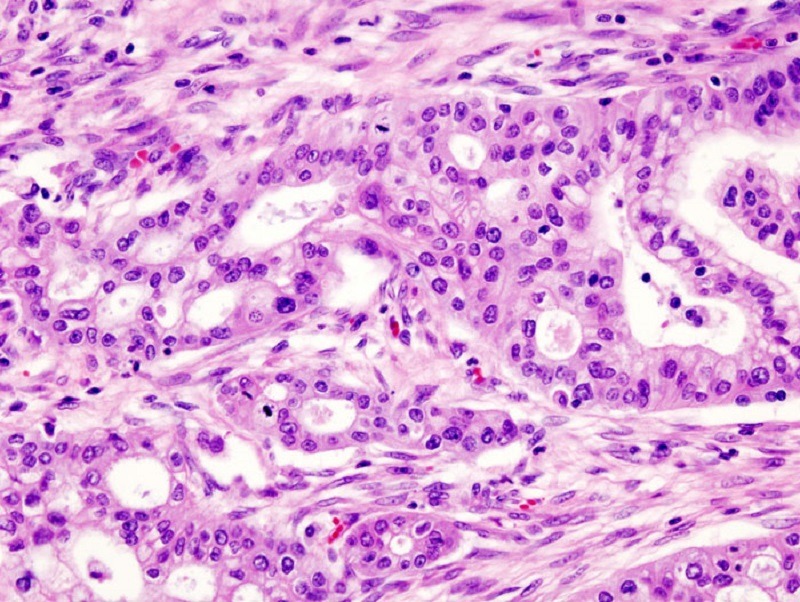
MAb-AR20.5 is an IgG1k type murine monoclonal antibody that attaches specifically to the circulating and tumour-associated antigen (MUC1) expressed on pancreatic cancer cells. It is said to become
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.