The US Food and Drug Administration (FDA) has agreed to review Emergent BioSolutions’ Biologics License Application (BLA) for AV7909 (Anthrax Vaccine Adsorbed, Adjuvanted).
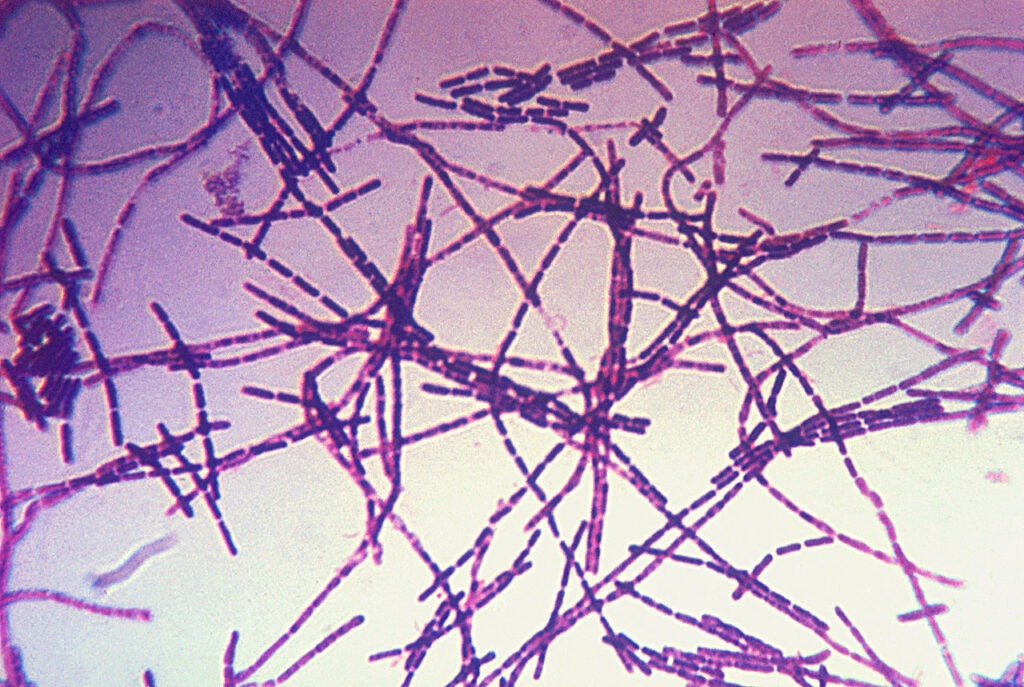
Photomicrograph of Bacillus anthracis bacteria using Gram-stain technique. Credit: Centers for Disease Control and Prevention/ commons.wikimedia.org.
Subscribe to our email newsletter
The vaccine candidate seeks to prevent anthrax in people after suspected or confirmed exposure to Bacillus anthracis. AV7909 is being assessed in adult persons between 18 and 65 years of age when administered in conjunction with recommended antibacterial drugs.
The company said that the Prescription Drug User Fee Act goal date, or the decision on approval, is expected in April next year.
Emergent BioSolutions research and development senior vice-president Kelly Warfield said: “Over the last 20 years, Emergent has partnered with the US government to lead this programme from early- to advanced-stage development.
“As we progress toward licensure of AV7909, which is designed to follow a two-dose immunisation schedule and to elicit a faster immune response, we redouble our efforts to support the government’s overall preparedness and response strategy for large-scale emergencies involving anthrax and other threats to public health.”
The rolling BLA submission was completed in April 2022. The submission is based on AV7909’s phase III clinical study that assessed the lot consistency, immunogenicity, and safety of the vaccine candidate.
During the trial, a two-dose regimen was administered intramuscularly in healthy adults.
Data from the phase II study were also included in the submission.
The company added that the BLA submission was completed under a contract to develop and deliver the vaccine candidate. This is funded by the Biomedical Advanced Research and Development Authority (BARDA) in the US.
Last month, FDA accepted the supplemental biologics license application (sBLA) for Sanofi and Regeneron’s Dupixent (dupilumab) for priority review.
 Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
Advertise With UsAdvertise on our extensive network of industry websites and newsletters.
 Get the PBR newsletterSign up to our free email to get all the latest PBR
news.
Get the PBR newsletterSign up to our free email to get all the latest PBR
news.

